Evaluating the behavior of cultured sertoli cells in the presence and absence of spermatogonial stem cell
Introduction
Spermatogonial stem cells (SSCs) are undifferentiated cells which are highly reproducible and expandable. These cells can generate completely similar cells (self-renewing); they can be differentiated and generate mature spermatozoa (1). It was recently shown that stem cells including SSCs are required to be in a specific tissue to express specific genes (2). In seminiferous tubules, SSCs are in near contact with Sertoli cells; sertoli cells produce suitable growth factors such as stem cell factors, transforming growth factor alpha and beta (TGF-β, TGF-α), insulin like growth factor1 (IGF1), fibroblast growth factor (FGF), epidermal growth factor (EGF) as well as hormones which regulate growth of male reproductive structures .In this context, GDNF and FGF2 are of the most important growth factors that play pivotal role in regulation of SSCs development (2,3). GDNF is a member of TGF-β family that contributes to SSCs self-renewal in different species of mammals including bovine (1,2,4). FGF2 stimulated germ cells proliferation and SSCs self-renewal in vitro (4) as well as in vivo (3). The removal of SSCs could help us know more about mechanisms by that niche cells to rebuild the germ cells after testicular damage, which could improve therapies to remake testicular germ cell in injury of testicular and germ cell evacuation (5). Accordingly, in the present research, the role of Sertoli cells on gene expression of GDNF and FGF2 during removal of SSCs from the in vitro culture was assessed (6). Hormonal control of spermatogenesis is through FSH and testosterone activity on Sertoli cells. FSH is an essential prerequisite for maintaining spermatogenesis in adult mammals (7,8). FSH stimulates GDNF production by the Sertoli cells and consequently increases the SSCs self-renewal (9).
Methods
The research was conducted in accordance with guidelines of the Animal Ethics Committee at the University of Tehran. The present study was conducted in the Department of Theriogenology, Faculty of Veterinary Medicine during February 2016.
Animals and testicular biopsy
Testicular biopsies were obtained from Holstein bull calves (n=4), aged between 3 to 5 months, as previously used by our lab (10). Sedation with Xylazine and local anesthesia with lidocaine were performed. The testicular biopsies samples were transferred to the laboratory in 15 mL tube containing Dulbecco’s Minimal Essential Medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA) with 10% Fetal Bovine Serum (FBS) (Sigma, USA) and 100 IU/mL penicillin-streptomycin (GIBCO, UK) on ice, within two hours.
Cell isolation
Cell isolation was performed using a two-step enzymatic isolation procedure as previously used by our lab (11). Briefly, the obtained testis tissue was washed three times in DMEM containing antibiotics. They were then minced into small pieces as much as possible by a sterile scissor. Then they were suspended in DMEM containing 1 mg/mL collagenase (Sigma-Aldrich, USA), 1 mg/mL hyaluronidase (Sigma-Aldrich, USA), 1 mg/mL trypsin (Sigma-Aldrich, USA) and 5 µg/mL DNase (Fermentas, Germany) at 37 °C in a shaker incubator for 60 minutes. After three times washing in DMEM, the digested interstitial cells were removed and seminiferous tubules were remained. During the second step of enzymatic digestion, the seminiferous tubules were again incubated at 37 °C in DMEM containing 1 mg/mL collagenase, 1 mg/mL hyaluronidase and 5 µg/mL DNase for 45 minutes. In this step seminiferous tubules were deconstructed and their cells were separated. Finally, obtained cellular suspension was centrifuged at 30 ×g for 2 minutes to achieve population individual cells. Following filtration through 77 and 55 mm nylon filters, the cells were pelleted. The pellet was re-suspended in the DMEM containing antibiotics and 10% FBS.
Cell culture
To evaluate gene expression, we used 6-well plates (TPP, Switzerland). Cells were seeded at concentration of 10×105 per well contacting DMEM and FSH (30 IU/mL). The plates were incubated at 37 °C in the presence of 5% CO2 for 15 days. DMEM (Sigma-Aldrich, St. Louis, MO, USA) containing 10% FBS (Sigma, USA), 100 IU/mL penicillin and 100 mg/mL streptomycin was used for culturing cells. In the germ cell-removed group, the SSCs were removed from the in vitro culture as described by He et al. (12). Three hours after incubation, somatic cells attached to the bottom of wells but SSCs had remained in the suspension and consequently they were removed from the culture medium by using sampler (12) and no intervention was performed in the control group. In two groups, culture medium plus FSH were refreshed every 3 days.
Cells identification
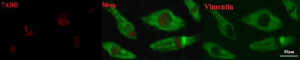
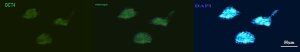
Vimentin is a cytoskeleton protein in Sertoli cell cytoplasm. At day 6 of culture, for Sertoli cells identification, Vimentin was stained, as described by Anway et al. (13) and Tajik et al. (14) and the specific marker Oct-4 was assessed in colonies of SSCs by the method proposed by Kubota et al. (4).
Evaluation of the colonization
Colonies in each well were counted in the control and germ cell-removed groups were assessed using an inverted microscope (IX71, Olympus, Japan).
Gene expression
Expression of the considered genes was assessed in the days 0, 6 and 12. Following trypsinization of the cultured cells (n=4 cell population from different calves), total RNA existing in the cells was extracted using Trizol (Fermentas, Germany). In order to prevent contamination of DNA, the extracted RNA was treated by DNase І (Fermentas, Germany). Concentration of the extracted RNA was determined by using spectrophotometry (Eppendorf, Germany). cDNAs were built by using 500 ng RNA extracted and oligo-primers and cDNA synthesis kit (Fermentas, Germany). Table 1 lists the primers of the considered genes. PCR was done by using SYBR Green master mix (Fermentas, Germany) and by thermocycler (Applied Biosystems, USA). PCR started with a primary melting stage for 5 minutes at 95 °C to activate polymerase and continued with 40 cycles including melting (30 s at 95 °C), synthesis (30 s at 58 °C) and formation (30 s at 72 °C). Quality of PCR reactions was determined by melting curve analysis. For each sample, PCR was done for reference gene (B-ACTIN) and target gene simultaneously. Cycle threshold (Ct) of the reference gene was subtracted from cycle threshold of the target gene to obtain ΔCt. In each interaction Ct on day zero was considered as a calibrator. Consequently, the relative gene expression was obtained by using Livak and Schmittgen (15) and calculation of ΔΔCt.

Full table
Statistical assessment
Data were analyzed statistically by using SPSS, version 24. Gene expression data was analyzed by using paired-samples t-test. Data was reported in the form of mean ± standard deviation. Differences were considered significant (P<0.05).
Results
Immunocytochemical staining of Sertoli cells and SSCs
Presence of the vimentin in sertoli cells was shown by immunocytochemistry staining (Figure 1) and Oct-4 was detected in the colonies of SSCs (Figure 2).
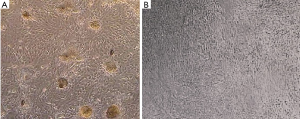
Colonization of SCCs
SSCs Colonies were developed in the control group (A) and no colony was observed in the germ cell-removed group (B) (Figure 3).
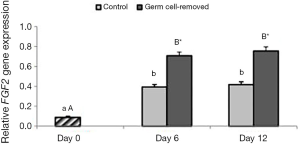
Gene expression
Expression of FGF2 significantly increased in both groups on days 6 and 12 compared to day 0 (P<0.0001). On days 6 and 12, expression of FGF2 was not different in group 1 and 2 (P>0.05). On days 6 and 12, expression of FGF2 was significantly higher in group 2 than group 1 (P<0.05) (Figure 4).
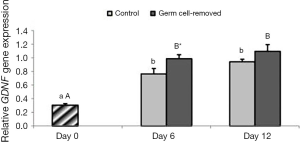
In two groups expression of GDNF significantly increased on days 6 and 12 compared to day 0 (P<0.05), while expression of GDNF was not different on days 6 and 12 (P>0.05). On day 6, expression of GDNF was higher in group 2 than group 1 (P<0.05), while it was not different in group 1 and 2 on day 12 (Figure 5).
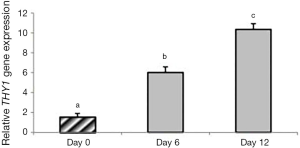
Expression of THY1 significantly increased in group 1 on day 6 (P=0.027) and 12 (P<0.0001) compared to day 0 (P<0.05). Moreover, a significant increase was observed on day 12 compared to day 6 (P=0.005). Expression of THY1 was not observed in group 2 (Figure 6).
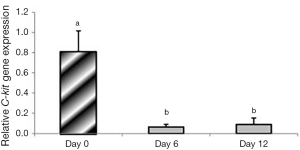
In group 1, expression of C-Kit significantly decreased on days 6 and 12 compared to day 0 (P<0.05); however, no significant difference was found between days 6 and 12 (P=0.396). Expression of C-Kit was not observed in group 2 (Figure 7).
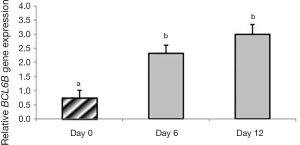
In group 1, expression of BCL6B was significantly higher on day 6 (P=0.004) and day 12 (P<0.0001) than day 0 (P<0.05). There was no significant difference on days 6 and 12 in expression of BCL6B in group 1 (P>0.05). Expression of BCL6B was not observed in group 2 (Figure 8).
Discussion
Colony formation, resulting from the interactions between SSCs and Sertoli cells during in vitro culture (16,17), were absent in the germ cell-removed group (18). In this context, THY1 (2,4,18) and BCL6B (4,8) are considered as undifferentiated markers of spermatogonia and expression of these markers has been reported in rich populations of SSCs. On the other hand, C-Kit is known as differentiated spermatogonial marker (19). Expression markers of spermatogonia, covering THY1, BCL6B and C-Kit (20,21), was not observed in the germ cell removed group. These findings showed that removal of SSCs was successfully performed in the present study. Vimentin is a cytoskeleton protein in Sertoli cell cytoplasm. For Sertoli cells identification, Vimentin was stained. This finding is similar to the previous studies by Anway et al. (13). For confirmation of the presence of SSCs, the specific marker Oct-4 was assessed in colonies of SSCs by the method proposed by Kubota et al. (4). Hence, the present study showed that SSCs removal increased the expression of GDNF during the culture. The recent studies showed that, sharp increase in GDNF expression stimulates self-renewal and inhibits differentiation of SSCs (2,22). On the other hand, undifferentiated SSCs gradually disappear in mice with deficient GDNF gene expression and only Sertoli cells remain in seminiferous tubules (2). In addition, assessment of GDNF expression has shown that GDNF plays a basic role in proliferation and differentiation of SSCs (19).
Studies have shown that addition of GDNF to culture leads to proliferation of SSCs (4,12). Hence, self-renewal of SSCs in ordinary culture can be increase expression of GDNF, as previously reported by He et al. (12). Expression of GDNF increased in response to removal of SSCs from the culture medium; this phenomenon may indicate reaction of Sertoli cells to rebuild reserves of testicular stem cells, has been reported by Tadokoro et al. (9) and Zohni et al. (23). Busulfan treatment has direct consequences of SSC loss and expansion, the testicular somatic environment responds rapidly and temporarily to the loss of spermatogonia, by Increasing expression of GDNF, after treatment with busulfan (23). Busulfan preferentially kills spermatogonia but it doesn’t affect on sertoli cell numbers. After busulfan treatment Sertoli cells have expressed GDNF in accordance the degree of damage on the spermatogonial population (24). Proliferation and self-renewal of SSCs stimulated by FGF2 (3,4). FGF2 and GDNF through upregulation of ETS variant have Synergistic effect (6) (Etv5), in addition GDNF signals increased expression of receptor tyrosine kinas Ret (3). Expression of FGF2 increased in two groups during culture. Therefore, it seems that the increase in expression of FGF2 is another reaction of Sertoli cells to rebuild testicular stem cells following the loss of testicular germ cells. This phenomenon, dominance of self-renewal on differentiation during the regeneration of germ cells, was previously observed in testis of mice treated with busulfan and lost a considerable part of their germ cells (25).
These finding could help develop therapies for acceleration of male fertility amendment after chemotherapy. Moreover it could improve in vitro condition for increase of self-renewal to SSCs transplantation. This research could be suggested that removal of SSCs could serve as a model to study the events ensuing testicular germ cell damage and the mechanisms involved in regeneration of testicular germ cells afterwards.
Conclusions
The present study showed that removal of SSCs from the culture medium could be a model for damage to SSCs; this is followed by upregulation of FGF2 and GDNF to stimulate self-renewal of SSCs and abrogation of differentiation. This reaction of somatic niche cells occurs to retrieve reserves of testicular germ cells in response to the damage.
Acknowledgements
We would also thank Dr. Arash Ghalyanchi, Dr. Golshid Javedani Shahedin and Dr. Saeed Farzad. The project was partly funded by stem Center of Excellence in Application of Stem Cells in Cell Therapy and Tissue Engineering, Faculty of Veterinary Medicine, University of Tehran.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The research was conducted in accordance with guidelines of the Animal Ethics Committee at the University of Tehran (7508016/6/25).
References
- Oatley JM, de Avila DM, Reeves JJ, et al. Testis tissue explant culture supports survival and proliferation of bovine spermatogonial stem cells. Biol Reprod 2004;70:625-31. [Crossref] [PubMed]
- Meng X, Lindahl M, Hyvönen ME, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000;287:1489-93. [Crossref] [PubMed]
- Ishii K, Kanatsu-Shinohara M, Toyokuni S, et al. FGF2 mediates mouse spermatogonial stem cell self-renewal via upregulation of Etv5 and Bcl6b through MAP2K1 activation. Development 2012;139:1734-43. [Crossref] [PubMed]
- Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A 2004;101:16489-94. [Crossref] [PubMed]
- Howell SJ, Shalet SM. Testicular function following chemotherapy. Hum Reprod Update 2001;7:363-9. [Crossref] [PubMed]
- Johnston H, Baker PJ, Abel M, et al. Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology 2004;145:318-29. [Crossref] [PubMed]
- Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol 2005;279:114-24. [Crossref] [PubMed]
- Sadate-Ngatchou PI, Pouchnik DJ, Griswold MD. Identification of testosterone-regulated genes in testes of hypogonadal mice using oligonucleotide microarray. Mol Endocrinol 2004;18:422-33. [Crossref] [PubMed]
- Tadokoro Y, Yomogida K, Ohta H, et al. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev 2002;113:29-39. [Crossref] [PubMed]
- Tajik P, Sani RN, Moezifar M, et al. Effect of follicle-stimulating hormone and testosterone on colony formation of bovine spermatogonial stem cell. Comp Clin Path 2014;23:901-6. [Crossref]
- Shafiei S, Tajik P, Ghasemzadeh-nava H, et al. Isolation of bovine spermatogonial cells and co-culture with prepubertal sertoli cells in the presence of colony stimulating factor-1. Iran J Vet Med 2013;7:83-90.
- He Z, Kokkinaki M, Jiang J, et al. Isolation, characterization, and culture of human spermatogonia. Biol Reprod 2010;82:363-72. [Crossref] [PubMed]
- Anway MD, Folmer J, Wright WW, et al. Isolation of Sertoli cells from adult rat testes: an approach to ex vivo studies of Sertoli cell function. Biol Reprod 2003;68:996-1002. [Crossref] [PubMed]
- Tajik P, Barin A, Movahedin M, et al. Nestin, a neuroectodermal stem cell marker, is expressed by bovine sertoli cells. Comp Clin Path 2012;21:395-9. [Crossref]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001;25:402-8. [Crossref] [PubMed]
- Aponte PM, Soda T, Van De Kant H, et al. Basic features of bovine spermatogonial culture and effects of glial cell line-derived neurotrophic factor. Theriogenology 2006;65:1828-47. [Crossref] [PubMed]
- Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol 2008;24:263-86. [Crossref] [PubMed]
- Skinner MK. Sertoli cell secreted regulatory factors. Sertoli Cell Biology 2005:107-20.
- Johnston DS, Olivas E, DiCandeloro P, et al. Stage-specific changes in GDNF expression by rat Sertoli cells: a possible regulator of the replication and differentiation of stem spermatogonia. Biol Reprod 2011;85:763-9. [Crossref] [PubMed]
- Izadyar F, den Ouden K, Creemers LB, et al. Proliferation and differentiation of bovine type A spermatogonia during long-term culture. Biol Reprod 2003;68:272-81. [Crossref] [PubMed]
- Reding SC, Stepnoski AL, Cloninger EW, et al. THY1 is a conserved marker of undifferentiated spermatogonia in the pre-pubertal bull testis. Reproduction 2010;139:893-903. [Crossref] [PubMed]
- Yomogida K, Yagura Y, Tadokoro Y, et al. Dramatic expansion of germinal stem cells by ectopically expressed human glial cell line-derived neurotrophic factor in mouse Sertoli cells. Biol Reprod 2003;69:1303-7. [Crossref] [PubMed]
- Zohni K, Zhang X, Tan S, et al. The efficiency of male fertility restoration is dependent on the recovery kinetics of spermatogonial stem cells after cytotoxic treatment with busulfan in mice. Hum Reprod 2012;27:44-53. [Crossref] [PubMed]
- Bucci LR, Meistrich ML. Effects of busulfan on murine spermatogenesis: cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutat Res 1987;176:259-68. [Crossref] [PubMed]
- Nakagawa T, Sharma M, Nabeshima Y, et al. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 2010;328:62-7. [Crossref] [PubMed]
Cite this article as: Jabarpour M, Tajik P. Evaluating the behavior of cultured sertoli cells in the presence and absence of spermatogonial stem cell. Stem Cell Investig 2018;5:1.


