Not just another biomarker: the role of integrin alpha 7 in glioblastoma
Glioblastoma is the most lethal primary brain tumor, and one of the most aggressive and invasive types of cancer overall. Despite treatment efforts, median length of survival for glioblastoma patients is between 12 and 18 months (1). Aside from their aggressive nature, glioblastomas are also known for being heterogeneous, with many different cell types throughout the tumor. Glioblastoma stem cells (GSCs) are one cellular subtype within these tumors and are characterized by unlimited self-renewal and resistance to treatment. Cancer stem cells have been identified in brain tumors as well as other cancers, and are thought to play a central role in the malignancy of these tumors (2,3). Hence, there is huge interest amongst researchers to find ways to target the cancer stem cell population specifically. Knowing which cells within a tumor are the extremely tumorigenic GSCs is not possible without reliable biomarkers. As mentioned previously, glioblastomas are heterogeneous within each individual tumor, but are also heterogeneous from one patient to the next. Many groups have identified markers of cancer stem cells that turn out to have greater representation in certain subtypes over others, such as CD44 in mesenchymal tumor regions, and CD133/Olig2 in proneural tumor regions (4-7). Despite the wide use of these surface epitopes as biomarkers, there is still controversy over whether or not they are uniformly reliable across patient samples (2).
To address this issue, Haas et al. used an innovative strategy of generating and screening a library of antibodies produced against the surface antigens of GSC neurospheres. This provided an unbiased platform to find antibodies that specifically bound GSCs and to then identify and interrogate the antigen they were binding as a novel marker of GSCs. Each antibody generated was tested for its ability to bind surface epitopes across GSC samples. Through this approach, the number of possible biomarkers was narrowed from 12,000 down to just 6. A final round of screening against primary GBM samples led to a final antibody of interest, named mAb 1.4A2. This approach easily allowed for identification of a target biomarker that was expressed in numerous GSC samples and not just one particular patient sample.
The authors then investigated whether this antibody could enrich for GSCs from bulk tumor samples using fluorescence activated cell sorting (FACS). Primary spheroid GBM cultures were designed to express GFP and FACS sorted from dissociated xenographs to exclude any non-tumor cells. The tumor cells were then further enriched by FACS for mAb1.4A2 high or low expression. The cells with high mAb1.4A2 expression were also positive for the validated markers of stem-like cells, Nestin and EphA2. On the other hand, the cells that had low mAb 1.4A2 levels expressed markers of differentiation. This implied that cells with higher expression of mAb 1.4A2 were those that were more stem-like. This finding was further supported by the fact that cells with high mAb 1.4A2 could initiate tumors in an orthotopic mouse model whereas the low mAb 1.4A2 cells did not. These results were crucial to conclude that the cells highly expressing mAb 1.4A2 were the most aggressive cells, and behaved in a way that was very characteristic of GSCs.
Now that it was established that mAb1.4A2 could identify cells with more stem-like characteristics, mass spectrometry and immunoprecipitation were used to identify the antigen in question. Two possible antigens were found: integrin alpha 7 (ITGA7) and integrin beta 1 (ITGB1). Cells were transduced with either ITGA7 or ITGB1 cDNA, and the antibody only bound to the ITGA7 expressers. ITGA7 naturally binds laminin in normal cells, and interestingly, when the anti-ITGA7 antibody was applied, it was able to block this function when cells were plated on a laminin matrix. Although it was not yet known if the function of ITGA7 was important to the stem-like properties of GSCs, ITGA7 had been identified as a target for further testing.
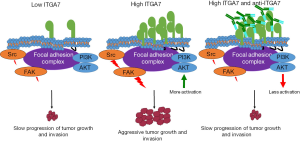
The authors next wanted to understand if ITGA7 expression in GSCs had a function, and if it was a possible target for therapy. They investigated pathways and targets within the cells crucial to GSC growth and invasion. Initial screens of proteins involved in tumor growth revealed no patterns amongst GSC patient samples. There appeared to be complete heterogeneity in protein activation between samples except that p38 was down-regulated in ITGA7 knockdown cells. They decided to look into other pathways as well, and found that cell cycle proteins and proteins in the PI3K/AKT pathway were altered by the inhibition of ITGA7; this effect was seen with both shRNA knockdown and treatment with anti-ITGA7. Furthermore, there was a decrease in phospho-FOXO3a levels, a downstream target of the AKT pathway, when anti-ITGA7 was administered. These data suggest that the AKT pathway is inhibited by anti-ITGA7. However, blocking this pathway did not lead to cell death. There was not an increase in caspase 3/7, but there was an increase in p27/Kip1, a cell cycle inhibitor. Because there were similar phenotypes to anti-ITGA7 when they treated GSCs with the ITGB1 shRNA, it is possible that blocking the ITGA7/B1 dimer may be able to block the cell cycle without inducing apoptosis.
In both in vitro and in vivo systems, the GSCs with depleted ITGA7 were less malignant than controls. Using a limiting dilution assay, they determined that ITGA7 depletion led to less clonogenic survival of GSCs. They then injected ITGA7 depleted GSCs into an orthotopic mouse model and saw either slow or no tumor growth in comparison to the aggressive growth with control GSCs. This suggested that ITGA7 expression was a predictor, and possibly a mechanism, for GSC-driven lethality. To take this idea one step further, the authors examined public databases to evaluate the ITGA7 expression in low- and high-grade gliomas. There was a significant correlation between high-grade tumors and higher expression of ITGA7, again corroborating the idea that ITGA7 is related to the GSC-driven lethality.
Many other groups have previously shown that the ITGA7/B1 dimer binds laminin naturally (8). Moreover, it has been demonstrated that these integrins are necessary for muscle cell migration and cell motility when on laminin (9). Expanding upon this, the authors felt that ITGA7 may be involved in the invasiveness of GSCs, and tested this by a laminin invasion assay whereby cells with depleted ITGA7 were much less invasive on the laminin. This result suggested that ITGA7 may be involved in invasion of GSCs. This finding, in and of itself, was striking and underscored that ITGA7 was more than just a biomarker as it was functionally important for cell invasiveness, a property that is integral to the deadly nature of glioblastomas.
While it was obvious that ITGA7 was likely involved in cell invasion, it was not clear how. To explore the mechanism for this phenomenon, the authors looked at signaling cascades that are downstream of integrins. GSCs were again grown on a laminin matrix (their natural binding substrate), and there was an increase in phosphorylated Src and focal adhesion kinase (FAK). This was intriguing as Src is a well-known oncogene and has been implicated in cell invasion and migration (10). FAK is also associated with cell invasion and has been linked to metastasis in other cancers (11). Importantly, activation of this signaling pathway was attenuated with the addition of the blocking antibody to ITGA7.
The final step was to validate the clinical relevance of targeting ITGA7. The researchers first injected luciferase-expressing GSCs expressing either high or low levels of ITGA7 subcutaneously into a mouse. The mice were then treated with anti-ITGA7 or isotype control. Anti-ITGA7 treatment showed a significant reduction in tumor growth, however the tumors did resume growing when the antibody treatment was discontinued. Of note, the tumors in anti-ITGA7 treated mice were still significantly smaller than isotype control four months after implantation. These data were promising, so the experiment was next done in an intracranial xenograph model. GSCs were orthotopically injected into mice that were then treated either with control antibody or anti-ITGA7. The mice treated with anti-ITGA7 survived significantly longer than those treated with control. The experiment was then conducted with GFP-expressing GSCs. The brains of these mice were analyzed post-mortem, and the results were again, striking. The mice who had received the antibody had significantly less GSC cell invasion, evident by a lack of GFP-expressing cells throughout the brain. In contrast, the vehicle animals showed cell invasion into many different brain structures.
The cancer stem cell field continues to provide a refined view of the functional diversity associated with the intracellular heterogeneity of tumors. This study makes a great contribution to the glioblastoma cancer stem cell field in particular as it has identified a more universal marker for GSCs with validated functional relevance that can be therapeutically targeted (Figure 1). As other integrins have also been shown to be crucial for GSCs, it may be beneficial to target multiple integrins and create a synergistic effect by capitalizing on multiple points of vulnerability for GSCs (12). Furthermore, ITGA7 may be critical to other tumors with a cancer stem cell component and therefore blocking ITGA7 may have a more universal clinical utility. Lastly, it would be very interesting to explore combinatorial therapy along with ITGA7 blocking antibodies to achieve an even greater therapeutic impact since it was demonstrated that tumor growth resumed with the antibody was stopped. Being a biomarker that is seen across many different patient samples makes ITGA7 a very promising area for future research, and one that hopefully will benefit future patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Lathia JD, Mack SC, Mulkearns-Hubert EE, et al. Cancer stem cells in glioblastoma. Genes Dev 2015;29:1203-17. [Crossref] [PubMed]
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature 2004;432:396-401. [Crossref] [PubMed]
- Brown DV, Filiz G, Daniel PM, et al. Expression of CD133 and CD44 in glioblastoma stem cells correlates with cell proliferation, phenotype stability and intra-tumor heterogeneity. PLoS One 2017;12:e0172791. [Crossref] [PubMed]
- Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006;9:157-73. [Crossref] [PubMed]
- Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010;17:98-110. [Crossref] [PubMed]
- Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014;344:1396-401. [Crossref] [PubMed]
- von der Mark H, Williams I, Wendler O, et al. Alternative splice variants of alpha 7 beta 1 integrin selectively recognize different laminin isoforms. J Biol Chem 2002;277:6012-6. [Crossref] [PubMed]
- Mielenz D, Hapke S, Pöschl E, et al. The integrin alpha 7 cytoplasmic domain regulates cell migration, lamellipodia formation, and p130CAS/Crk coupling. J Biol Chem 2001;276:13417-26. [Crossref] [PubMed]
- Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene 2004;23:7906-9. [Crossref] [PubMed]
- Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 2014;14:598-610. [Crossref] [PubMed]
- Lathia JD, Gallagher J, Heddleston JM, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 2010;6:421-32. [Crossref] [PubMed]
Cite this article as: Zalenski A, De K, Venere M. Not just another biomarker: the role of integrin alpha 7 in glioblastoma. Stem Cell Investig 2017;4:99.


