Breast cancer stem cells—from origins to targeted therapy
Introduction
Breast cancer is one of the most common causes of cancer-related deaths in women worldwide (1). Gratifyingly, improvement in recent advances of breast cancer screening methods and treatment strategies such as chemotherapy and radiotherapy contribute to a significant eradication of primary tumour bulk, thereby increasing chances of survival for breast cancer patients (1). However, despite available interventions, patients under remission may still develop breast cancer relapse and metastasis (2,3). Accumulating evidence suggests that the underlying presence of a small subpopulation of undifferentiated cells, termed breast cancer stem cells (BCSCs), are most likely give rise to tumour progression, spreading and resistance to conventional therapy (2,4).
However, the real nature of BCSCs remains unclear. Much research is still ongoing to foster a deeper understanding of BCSCs on the formation of breast cancer which is essential for pursuing new therapeutic strategies and to improve diagnosis and prognosis for breast cancer patients. Hence, this article aims to discuss the current literature on the origin of BCSCs, stem cell biomarkers for identification and development of new targeted therapy strategies that are available for breast cancer treatment.
Origin of BCSCs
The origin of BCSCs has stirred much controversy among researchers. Current experimental evidence proposed different theories about the origin of BCSCs, in which stem cells, progenitor cells or differentiated cells can be a potential model for BCSC formation (Figure 1).
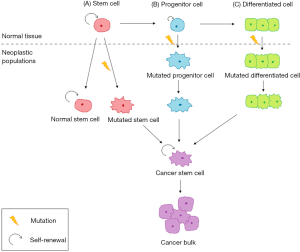
The concept of BCSCs arising from either mammary stem cells or progenitor cells seems more plausible among various hypotheses (5,6). Most supporting evidence shows similar phenotypic features and cell surface markers which are related to those specific cells originate from the same lineage in the differentiation hierarchy. Recent research identified that the CD44+CD24− cell marker expressed on mammary progenitor cells resemble the CD44+CD24−Lineage− found on BCSCs (6). Besides, the population of BCSCs also shared specific properties highly similar to normal mammary stem cells or partially differentiated mammary progenitor cells (7). They are characterised with the ability to undergo self-renewal, differentiation, tumour-initiating ability, invasion and resistance to conventional therapy which lead to generation of more cancer stem cells (CSCs) and heterogeneity of malignancy. Apart from that, due to the long-lived nature of stem cells, normal stem cells tend to persist in tissue for a longer period as compared with differentiated cells, which constantly undergo cellular turnover. Therefore, stem cells are more likely to acquire multiple genetic alterations which are crucial for oncogenic transformation.
Contrary to previous concepts, another school of thought suggests that the BCSC model can be derived from non-stem cells—differentiated mammary cells. Exposure to damaging environmental factors including chemotherapy and radiotherapy lead to genetics and heterotypic alterations of non-malignant somatic cells and hence causing de novo generation of CSC in which those cells undergo de-differentiation to regain its stem-like properties, which then leads to enrichment of BCSCs (8,9). Emerging evidence also suggests that microenvironment stimuli can trigger malignant transformation of differentiated cells into BCSCs (10). Regardless of all those different theories proposed, to date, there has been no concrete evidence to confirm the origin of BCSCs.
Biomarkers for isolating BCSCs
Identification of biomarkers is a critical step in defining BCSCs. The study of molecular signatures contributes to the characterization and isolation of BCSC subpopulations. A better understanding of stem cell markers expressed in breast cancer provide a better insight onto BCSC biology, and thus enable the discovery of new therapeutic targets. The most common biomarkers used to identify the BCSC phenotype are CD44, CD24, and ALDH1 (11). Other, less renowned biomarkers are also discussed (Table 1).
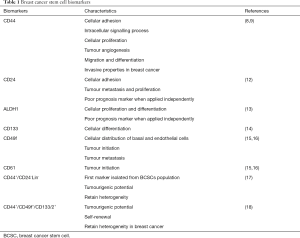
Full table
CD44
CD44 is a transmembrane glycoprotein present on the cell surface which plays an important role in adhesion, intracellular signalling, enhancing cell proliferation, tumour angiogenesis, differentiation, modulating migration and invasive properties in breast cancer (8,9). CD44 shows strong expression in BCSCs as well as numerous human cancers. CD44 acts to retain tumourigenicity and multi-potency of the population (19). Another study showed that CD44 interacts with hyaluronic acid to promote cell invasiveness and metastasis, however the mechanism remains unknown (20). Pham et al. [2011] demonstrated that BCSCs were able to differentiate into normal cells when the expression of CD44 decreases (21). Also, inhibition of CD44 expression decreases anti-tumour drug resistance (19).
CD24
CD24 is also a cell surface glycoprotein which enhances adhesion properties and promotes tumour metastasis and proliferation (22). Conversely, a study proved that upregulation of CD24 was capable to inhibit stemness in breast cancer cells (22). CD24 was found to express in a wide variety of cancers. Ahmed and colleagues [2012] showed that expression of CD24 was not associated with aggressive breast cancer subpopulation (23). Hence, this marker was considered a poor prognostic tool for identifying breast cancer when evaluated independently (12).
ALDH1
Aldehyde dehydrogenase (ALDH) is a form of detoxifying enzyme that catalyses oxidation of intracellular aldehydes and mediates conversion of retinol to retinoic acids, which then act as a cell proliferation modulator. Moreb et al. [2012] revealed that ALDH was found to mark both normal and cancerous mammary cells as assessed by the ADELFLUOR assays technique, and exhibit functional role in cell proliferation, differentiation and self-protection (13). Overexpression of ALDH1 leads to chemoresistance (13).
Other biomarkers
The other biomarkers involved in identification of BCSCs population includes CD133 which is found in triple-negative breast cancer and BRCA-1 tumours (14,24). The specific function of CD133 expression in cancer cells has not been defined, but it is known to be associated with cholesterol binding, and thus suspected to be involved in Hedgehog (Hh) signalling responsible for cell differentiation and epithelial-mesenchymal transition (24). In addition, Desgrosellier et al. [2014] demonstrated that CD49f and CD61 were found to be associated with tumour initiation properties through an in-vivo study of breast cancer in mice (15,16).
Combinatorial expression
Evaluation of combinatorial expression of surface markers has been proven to yield a better prognostic value for identifying BCSCs. In a study done by Al-Hajj and his coworkers [2003], a subpopulation of human BCSCs exhibiting the CD44+/CD24−Lin− phenotype had been identified (17). Cells expressing this phenotype show strong tendency to transform into CSCs. However, not all are associated with aggressive metastatic growth (2). More recently, CD44+/CD49f+/CD133/2+ was found to demonstrate increased tumourigenic potential, self-renewal and heterogeneity in breast cancer (18).
Signaling pathways regulating BCSCs
Notch, Hh and Wnt pathways are essential signalling pathways that are responsible for the normal process of tissue maintenance. Any deregulation of these pathways in mammary glands may lead to transformation of normal stem cells into CSCs.
The Notch signalling pathway is responsible for the regulatory process of self-renewal and cellular differentiation during the developmental stage of cells (25). Since the Notch pathway mainly targets genes with high proliferation and apoptosis inhibition properties, therefore activation of this pathway in breast cancer results in uncontrolled development and maintenance of BCSCs. D’Angelo et al. [2015] showed malignant transformation of cells when Notch-4 activity was increased in several in vitro studies (26). A recent study demonstrated the application of antibodies specifically towards Notch receptors enable inhibition of tumour growth (27). Furthermore, Simmons et al. [2012] also showed that inhibiting Notch-1 is capable of inducing tumour regression in a mouse mammary tumour model (28).
The Hh pathway is associated with tissue patterning, development and progression. Increased activation of the Hh pathway has been identified in several CSCs models including breast cancer (29,30). Deregulation of the Hh pathway initiates increased expression of Sonic hedgehog-(Shh), one of the ligands in the Hh pathway or Gli1, a downstream transcription factor in human breast cancer that supports the development and progression of breast cancer by promoting angiogenesis (29,31). In the same study, silencing CYR61 from Shh-expressing Hh cells inhibited the malignant behaviour of tumour cells, leading to limited vasculature and metastasis (31).
The Wnt signalling pathway plays a crucial role in regulating stem cell division and self-renewal. Activation of Wnt/β-catenin signalling pathway initiates stem cell and progenitor cell proliferation, hence resulting in an increase of mammary tumour bulk. The Wnt pathway was also found to aid in chemoresistance and radioresistance of BCSCs (32). Tumour progression can be curbed by suppressing the activation of Wnt/β-catenin pathway. Jang et al. [2015] revealed potential therapeutic advantages of shRNA-mediated Wnt1 silencing (33). Suppression of Wnt protein leads to a significant decrease in stem cell marker expression, and in turn indicating the reduction of BCSC population. Such inhibition can restrain self-proliferation and migration of CSCs, suggesting that targeting the Wnt/β-catenin pathway could be a key therapeutic approach in removing BCSCs.
These three pathways play essential roles in regulating BCSC self-renewal, and are similarly involved in normal stem cell development. From a clinical prospective, further studies are required to elucidate the mechanisms regulating BCSCs, in order to achieve the desired elimination of BCSCs without ablating normal cell function.
MicroRNAs regulating BCSCs
MicroRNAs-(miRNAs) are short, non-coding regulatory ribonucleic acids (RNAs) that aid in regulating crucial biological processes and usually deregulated in cancer. Recently, numerous miRNAs are revealed to be upregulated or downregulated in breast cancer. Deregulation of miRNAs expression associated with carcinogenesis and drug resistance in BCSC populations (Table 2).

Full table
Loss of miR200c is associated with tumourigenicity of BCSCs and normal mammary stem cells. Jurmeister et al. [2012] revealed that downregulation of miR-200c helps in triggering breast cancer cells to invade and migrate (34). Hence, a future treatment for metastatic progression of breast cancer can be developed by re-expressing miR-200c.
Besides that, suppression of miR-205 in BCSC populations showed drug resistance properties (35). MiR-141 is also downregulated in BCSCs, which contributes to dedifferentiation of breast cancer cells which in turn enhances the population (36). Decreased expression of miR-34a in human mammary cancer results in suppression of stem cell properties. Study from Kang et al. [2015] revealed that miR-34a targeted the involvement of the Notch-1 pathway in maintaining stem cell properties of BCSC populations, hence suggesting that the miR-34a/Notch-1 pathway may be a potential therapeutic target for treating breast cancer (41).
Apart from that, elevated expression of let-7 miRNA is involved in carcinogenesis and tumour development of BCSCs. Sun et al. [2016] discovered that isoform let-7c interrelated with Wnt signalling pathway in vivo to regulate BCSC renewal (42). Furthermore, miR-1 also associated with Wnt signalling pathway which is crucial for aggressiveness of breast cancer (37). Most of these findings provide a reference for the future development of miRNA-based therapy. However, further studies are still needed required to target the mechanism regulating breast cancer stemness.
Targeted therapy of BCSCs
Most conventional therapies currently available are capable of eliminating primary tumour bulk, but may not be able to provide durable clinical results in treated patients. This is because conventional therapy has limited application toward eliminating BCSC population, thus providing an opportunity for breast cancer to relapse. Therefore, a new targeted therapeutic strategy that aim for specific biomarkers, signalling pathways and microRNAs has been developed to provide a safer and more effective treatment selectively for breast cancer.
Nanoparticles that encapsulate low dose decitabine was developed by Li and colleagues [2015] to sensitize chemotherapeutic response of CSC populations with high ALDH activity (43). In the same study, combined treatment of nanoparticles loaded with low dose decitabine and doxorubicin showed significant decreased of CSC population with high ALDH expression in vitro and showed increased sensitivity of BCSCs towards administered drugs (43). In addition, application of novel multifunctionalized iron oxide magnetic nanoparticles (MNPs) with anti-CD44 antibody and gemcitabine derivatives showed significant effects on CD44 positive cancer cells (44). Since BCSCs overexpressed CD44 biomarkers as previously mentioned, MNP is a potential approach for eliminating BCSCs.
Besides targeting biomarkers, nanoparticles also affect signalling pathways regulating CSCs. Nanoparticle-specific therapy has been designed to target specific signalling pathways regulating the stemness of BCSCs populations such as Wnt/β-catenin, Notch (45), and Hh pathway. Application of nanoparticle drug delivery system promises higher efficiency, lesser side effects and more CSC specificity towards treatment, however extensive research is still required to ensure safety for in vivo applications.
Conclusions and future directions
Over the decades, accumulating studies have strengthened the concept of breast cancer as a disease of BCSCs. In fact, plasticity of BCSCs plays a vital role in determining the evolution of disease. Targeting BCSCs possesses a great implication on new therapeutic strategies development which offers long-lasting disease remission and long-term survival of breast cancer patients.
However, the theory of BCSC origin has not been proven to explain cancer initiation. Also, there is no universal biomarker that is specific for identification of breast cancer. Unique tumourigenic mechanisms operating within BCSCs to make it distinct from the tumour bulk is yet to be investigated. Nevertheless, extensive study has to be done towards a new therapeutic approach in the form of nanoparticles, to ensure the safety of application in vivo. CSC research no doubt enables better understanding towards the nature of BCSC which can greatly aid in development of new therapeutic targets and enhance current therapeutic strategies.
Acknowledgements
The authors would like to thank the authorities of International Medical University, Malaysia, for provision of necessary facilities in the preparation of this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Lin Y, Zhong Y, Guan H, et al. CD44+/CD24- phenotype contributes to malignant relapse following surgical resection and chemotherapy in patients with invasive ductal carcinoma. J Exp Clin Cancer Res 2012;31:59. [Crossref] [PubMed]
- Zielske SP, Spalding AC, Wicha MS, et al. Ablation of Breast Cancer Stem Cells with Radiation. Transl Oncol 2011;4:227-33. [Crossref] [PubMed]
- Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res 2008;10:R25. [Crossref] [PubMed]
- Bao L, Cardiff RD, Steinbach P, et al. Multipotent luminal mammary cancer stem cells model tumor heterogeneity. Breast Cancer Res 2015;17:137. [Crossref] [PubMed]
- Liu S, Cong Y, Wang D, et al. Breast Cancer Stem Cells Transition between Epithelial and Mesenchymal States Reflective of their Normal Counterparts. Stem Cell Reports 2013;2:78-91. [Crossref] [PubMed]
- Ma R, Bonnefond S, Morshed SA, et al. Stemness is Derived from Thyroid Cancer Cells. Front Endocrinol (Lausanne) 2014;5:114. [Crossref] [PubMed]
- Lagadec C, Vlashi E, Della Donna L, et al. Radiation-Induced Reprogramming of Breast Cancer Cells. Stem Cells 2012;30:833-44. [Crossref] [PubMed]
- Koren S, Reavie L, Couto JP, et al. PIK3CAH1047R induces multipotency and multi-lineage mammary tumours. Nature 2015;525:114-8. [Crossref] [PubMed]
- Chaffer CL, Marjanovic ND, Lee T, et al. Poised Chromatin at the ZEB1 Promoter Enables Breast Cancer Cell Plasticity and Enhances Tumorigenicity. Cell 2013;154:61-74. [Crossref] [PubMed]
- de Beça FF, Caetano P, Gerhard R, et al. Cancer stem cells markers CD44, CD24 and ALDH1 in breast cancer special histological types. J Clin Pathol 2013;66:187-91. [Crossref] [PubMed]
- Kim HJ, Kim M-J, Ahn SH, et al. Different prognostic significance of CD24 and CD44 expression in breast cancer according to hormone receptor status. The Breast 2011;20:78-85. [Crossref] [PubMed]
- Moreb JS, Ucar D, Han S, et al. The enzymatic activity of human aldehyde dehydrogenases 1A2 and 2 (ALDH1A2 and ALDH2) is detected by Aldefluor, inhibited by diethylaminobenzaldehyde and has significant effects on cell proliferation and drug resistance. Chem Biol Interact 2012;195:52-60. [Crossref] [PubMed]
- Liu TJ, Sun BC, Zhao XL, et al. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene 2013;32:544-53. [Crossref] [PubMed]
- Lo PK, Kanojia D, Liu X, et al. CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin–TGFβ signaling. Oncogene 2012;31:2614-26. [Crossref] [PubMed]
- Desgrosellier JS, Lesperance J, Seguin L, et al. Integrin αvβ3 Drives Slug Activation and Stemness in the Pregnant and Neoplastic Mammary Gland. Dev Cell 2014;30:295-308. [Crossref] [PubMed]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci 2003;100:3983-8. [Crossref] [PubMed]
- Meyer MJ, Fleming JM, Lin AF, et al. CD44posCD49fhiCD133/2hi Defines Xenograft-Initiating Cells in Estrogen Receptor-Negative Breast Cancer. Cancer Res 2010;70:4624-33. [Crossref] [PubMed]
- Van Phuc P, Nhan PL, Nhung TH, et al. Downregulation of CD44 reduces doxorubicin resistance of CD44CD24 breast cancer cells. Onco Targets Ther 2011;4:71-8. [Crossref] [PubMed]
- Okuda H, Kobayashi A, Xia B, et al. Hyaluronan Synthase HAS2 Promotes Tumor Progression in Bone by Stimulating the Interaction of Breast Cancer Stem-Like Cells with Macrophages and Stromal Cells. Cancer Res 2012;72:537-47. [Crossref] [PubMed]
- Pham PV, Phan NL, Nguyen NT, et al. Differentiation of breast cancer stem cells by knockdown of CD44: promising differentiation therapy. J Transl Med 2011;9:209. [Crossref] [PubMed]
- Schabath H. CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. J Cell Sci 2006;119:314-25. [Crossref] [PubMed]
- Ahmed MA, Aleskandarany MA, Rakha EA, et al. A CD44−/CD24+ phenotype is a poor prognostic marker in early invasive breast cancer. Breast Cancer Res Treat 2012;133:979-95. [Crossref] [PubMed]
- Wright MH, Calcagno A, Salcido CD, et al. Brca1 breast tumors contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res 2008;10:R10. [Crossref] [PubMed]
- Zhou W, Wang G, Guo S. Regulation of angiogenesis via Notch signaling in breast cancer and cancer stem cells. Biochim Biophys Acta 2013;1836:304-20. [PubMed]
- D’Angelo RC, Ouzounova M, Davis A, et al. Notch Reporter Activity in Breast Cancer Cell Lines Identifies a Subset of Cells with Stem Cell Activity. Mol Cancer Ther 2015;14:779-87. [Crossref] [PubMed]
- Falk R, Falk A, Dyson MR, et al. Generation of anti-Notch antibodies and their application in blocking Notch signalling in neural stem cells. Methods 2012;58:69-78. [Crossref] [PubMed]
- Simmons MJ, Serra R, Hermance N, et al. NOTCH1 inhibition in vivo results in mammary tumor regression and reduced mammary tumorsphere-forming activity in vitro. Breast Cancer Res 2012;14:R126. [Crossref] [PubMed]
- O’Toole SA, Machalek DA, Shearer RF, et al. Hedgehog Overexpression Is Associated with Stromal Interactions and Predicts for Poor Outcome in Breast Cancer. Cancer Res 2011;71:4002-14. [Crossref] [PubMed]
- Tao Y, Mao J, Zhang Q, et al. Overexpression of Hedgehog signaling molecules and its involvement in triple-negative breast cancer. Oncol Lett 2011;2:995-1001. [PubMed]
- Harris LG, Pannell LK, Singh S, et al. Increased vascularity and spontaneous metastasis of breast cancer by hedgehog signaling mediated upregulation of cyr61. Oncogene 2012;31:3370-80. [Crossref] [PubMed]
- Zhang ZM, Wu JF, Luo QC, et al. Pygo2 activates MDR1 expression and mediates chemoresistance in breast cancer via the Wnt/β-catenin pathway. Oncogene 2016;35:4787-97. [Crossref] [PubMed]
- Jang GB, Kim JY, Cho SD, et al. Blockade of Wnt/β-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci Rep 2015;5:12465. [Crossref] [PubMed]
- Jurmeister S, Baumann M, Balwierz A, et al. MicroRNA-200c Represses Migration and Invasion of Breast Cancer Cells by Targeting Actin-Regulatory Proteins FHOD1 and PPM1F. Mol Cell Biol 2012;32:633-51. [Crossref] [PubMed]
- Hu Y, Qiu Y, Yagüe E, et al. miRNA-205 targets VEGFA and FGF2 and regulates resistance to chemotherapeutics in breast cancer. Cell Death Dis 2016;7:e2291. [Crossref] [PubMed]
- Finlay-Schultz J, Cittelly DM, Hendricks P, et al. Progesterone downregulation of miR-141 contributes to expansion of stem-like breast cancer cells through maintenance of progesterone receptor and Stat5a. Oncogene 2015;34:3676-87. [Crossref] [PubMed]
- Liu T, Hu K, Zhao Z, et al. MicroRNA-1 down-regulates proliferation and migration of breast cancer stem cells by inhibiting the Wnt/β-catenin pathway. Oncotarget 2015;6:41638-49. [Crossref] [PubMed]
- Li B, Lu Y, Wang H, et al. MiR-221/222 enhance the tumorigenicity of human breast cancer stem cells via modulation of PTEN/Akt pathway. Biomed Pharmacother 2016;79:93-101. [Crossref] [PubMed]
- Hui C, Yujie F, Lijia Y, et al. MicroRNA-34a and microRNA-21 play roles in the chemopreventive effects of 3,6-dihydroxyflavone on 1-methyl-1-nitrosourea-induced breast carcinogenesis. Breast Cancer Res 2012;14:R80. [Crossref] [PubMed]
- Han M, Liu M, Wang Y, et al. Antagonism of miR-21 Reverses Epithelial-Mesenchymal Transition and Cancer Stem Cell Phenotype through AKT/ERK1/2 Inactivation by Targeting PTEN. PLoS One 2012;7:e39520. [Crossref] [PubMed]
- Kang L, Mao J, Tao Y, et al. MicroRNA-34a suppresses the breast cancer stem cell-like characteristics by downregulating Notch1 pathway. Cancer Sci 2015;106:700-8. [Crossref] [PubMed]
- Sun X, Xu C, Tang SC, et al. Let-7c blocks estrogen-activated Wnt signaling in induction of self-renewal of breast cancer stem cells. Cancer Gene Ther 2016;23:83-9. [Crossref] [PubMed]
- Li SY, Sun R, Wang HX, et al. Combination therapy with epigenetic-targeted and chemotherapeutic drugs delivered by nanoparticles to enhance the chemotherapy response and overcome resistance by breast cancer stem cells. J Control Release 2015;205:7-14. [Crossref] [PubMed]
- Aires A, Ocampo SM, Simões BM, et al. Multifunctionalized iron oxide nanoparticles for selective drug delivery to CD44-positive cancer cells. Nanotechnology 2016;27:065103. [Crossref] [PubMed]
- Mamaeva V, Niemi R, Beck M, et al. Inhibiting Notch Activity in Breast Cancer Stem Cells by Glucose Functionalized Nanoparticles Carrying γ-secretase Inhibitors. Mol Ther 2016;24:926-36. [Crossref] [PubMed]
Cite this article as: Sin WC, Lim CL. Breast cancer stem cells—from origins to targeted therapy. Stem Cell Investig 2017;4:96.


