Stem-cell extracellular vesicles and lung repair
Stem cells and lung repair
Cell-based therapies have been widely used in experimental and clinical studies as a new therapeutic approach for lung diseases, since they modulate inflammation and affect the remodeling process (1).
The main source of adult stem cells for cell therapy is the bone marrow. Hematopoietic stem cells have been studied due to their capacity to differentiate into immune cells and modulate immune-cell proliferation and activity (1,2). Mesenchymal stem (or, more properly, stromal) cells (MSCs) are also present in bone marrow, and play a primary role in stimulating the maintenance, growth, and survival of other cells. MSCs are multipotent and have been found in many sources, such as adipose tissue, amniotic fluid, cord blood, and the lungs (3). Beyond their stromal properties, MSCs are known to have plastic capacity and immunomodulatory, antifibrotic, and microbicidal properties (4), which has motivated many groups to study their therapeutic potential in experimental lung diseases and test them in phase I and II trials for acute respiratory distress syndrome (ARDS) (5-7), chronic obstructive pulmonary disease (COPD) (8-10), silicosis (11), and idiopathic pulmonary fibrosis (IPF) (12).
The mechanisms by which MSCs might mitigate inflammation and injury are not completely understood, and likely involve multiple pathways mediated by the release of soluble mediators, extracellular vesicles (EVs), and/or organelle transfer, as well as through cell-to-cell contact (1,4). Secretory mediators were first proposed as a mechanism of action for stem cells because very few stem cells engraft after injection into recipient animals (13). Recent data from a variety of preclinical lung disease models, including ARDS, asthma, emphysema, and pulmonary arterial hypertension (PAH), have demonstrated that systemic administration of conditioned media (CM) obtained from MSCs alone can lead to protective effects similar to those of MSCs (14-21). Recent data suggest that EVs, also known as exosomes, microvesicles, or microparticles, which are released by the MSCs and present in CM, may yield beneficial effects (21-26). However, the specific mediators responsible, such as soluble proteins, EV components, or other components of the CM, have not yet been identified, and are likely to be different depending on the lung injury model (22). Information has emerged regarding the roles of specific miRNAs and other EV components as mediators of the protective effects of MSC administration in preclinical lung disease models, but much remains unknown (21-26).
EVs
Recently, in order to standardize the nomenclature, the International Society for Extracellular Vesicles established a definition of EVs and the minimal experimental requirements for research about EVs (27). EVs are defined as small membrane vesicles, which includes exosomes, microvesicles and apoptotic bodies. They are distinguished by specific membrane markers, origin, and size (exosomes, 40–150 nm; microvesicles, 0.1–2 µm; apoptotic bodies, 1–4 µm). Because of the overlapping sizes and lack of specific markers for each EV component, the International Society for Extracellular Vesicles recommends use of the term EVs to describe all types of such vesicles. EVs are considered mediators of intercellular communication, as they contain several proteins, microRNAs, mRNAs, long noncoding RNAs, lipid mediators, and even organelles with biological relevance (28).
A wide variety of cell types have been shown to release EVs, including immune cells, epithelial cells, endothelial cells, and tumor cells. EVs have been isolated and characterized from different body fluids, such as plasma, urine, and bronchoalveolar lavage fluid (BALF). Of interest to respiratory medicine, EVs are reportedly released from both immune and structural cells in the lungs, and have recently been reported to play a role in pathophysiology of asthma (29), COPD (30), and pulmonary artery hypertension (29,31). Potential applications of EVs as biomarkers for lung diseases and novel therapeutic targets have emerged (32-35). In this line, MSC-derived EVs can be an important tool for obtaining the clinical benefits of MSC treatment (22). Recently, a Good Manufacturing Practices-grade standard protocol for obtaining exclusively human MSC-derived EVs was proposed (36). The characterization and establishment of MSC-derived EVs will help to identify active components in therapeutic EVs for future clinical applications.
Effects of EVs in vitro
A significant body of literature obtained in animal models of inflammation has shown that stem cell-derived vesicles are also immunosuppressive, probably through the transfer of both RNA and proteins carried by EVs (37-41). EVs derived from MSCs exposed to normoxic or hypoxic conditions are efficiently internalized by bone marrow macrophages, eliciting their switch from M1 to M2 phenotype, downregulating interleukin (IL)-6 and nitric oxide synthase, and upregulating arginase 1 and chitinase-like 3 protein—typical markers of alternative macrophage activation (37). Mechanistically, it has been shown that the MSC exosomal miR-146a, a well-known anti-inflammatory microRNA, when transferred to macrophages, results in M2 polarization and increases survival in septic mice (42). In lipopolysaccharide-primed human monocytes, EV transfer of microRNAs and mitochondria have restored intracellular ATP, reduced levels of pro-inflammatory mediators, and greatly increased their phagocytic properties (43,44). Moreover, MSC-EV treatment induces tolerogenic signaling through promotion of T regulatory cells, apoptosis of effector T cells, and an increase in immunosuppressive cytokine IL-10 concentration when co-cultured with T lymphocytes (39,45).
EVs are taken up by other cell types. Injured human monocytes, as well as alveolar epithelial cells, uptake hMSC-derived EVs through the CD44 receptor (44). MSC exosomes obtained from human umbilical cord MSCs act directly on hypoxic vascular endothelial cells, inhibiting STAT3 signaling (23). Moreover, EVs have strong antiapoptotic and pro-proliferative effects in vitro (46-48). Finally, MSC-derived EVs have been implicated in the tissue-restoring effects of MSCs, including wound healing (49), antioxidant and antitumor effects (50), and microbicidal activity (51) (Figure 1). In short, EVs released from MSCs, which can be rapidly isolated by ultracentrifugation and filtration, exhibit anti-inflammatory properties, decrease oxidative stress, increase ATP, reduce alveolar edema, and can promote bacterial clearance. These properties suggest they could be safely and easily used for therapy of lung diseases.
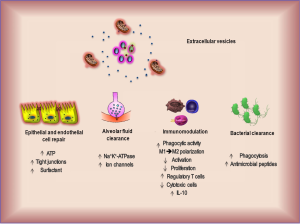
Effects of EVs in vivo
Stem cell-derived EVs have been tested in experimental lung injury, including models of asthma, ARDS, COPD, IPF, pneumonia, pulmonary artery hypertension, and silicosis (Table 1).
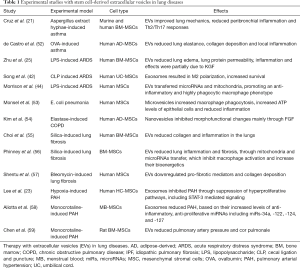
Full table
Asthma
Asthma is a common respiratory disease (affecting 1–18% of the population in different countries) usually characterized by chronic airway inflammation. It is defined by a history of respiratory symptoms such as wheezing, shortness of breath, chest tightness, and cough, which vary over time and in intensity, as well as variable expiratory airflow limitation (60). Among patients with asthma, 5–10% have severe disease, a result of mixed Th2/Th17-mediated neutrophilic airway inflammation. These patients experience poor clinical control and are resistant to corticosteroids and most other available treatments. Hence, new therapeutic options are still needed (61).
In this context, an increasing number of studies on cell therapy have demonstrated beneficial effects of systemic or local administration of syngeneic, allogeneic, or xenogeneic MSCs derived from bone marrow, adipose tissue, placenta, and other sources in a wide spectrum of preclinical asthma models. Therapy with MSCs during either antigen sensitization or challenge mitigates both airway hyperresponsiveness and lung inflammation in a variety of asthma models (21,52,62-74).
There is a growing experience demonstrating the benefit of MSC-derived EV therapy in experimental asthma (21,52). When administered systemically, both CM and, in particular, EVs isolated from human and murine bone marrow-derived MSCs at the onset of antigen challenge in previously sensitized mice were as potent as MSCs themselves in mitigating Th2/Th17-mediated allergic airway inflammation in a mouse model of severe refractory clinical asthma. Human MSCs (hMSCs), CM, and EVs were effective in this immunocompetent mouse model, ameliorating Aspergillus hyphae extract-provoked increases in airway hyperreactivity, lung inflammation, and the antigen-specific cluster of differentiation (CD)-4 T-cell T helper (Th)-2 and Th17 phenotype. Notably, both CM and EVs from hMSCs were generally more potent than those from mouse MSCs (mMSCs) in most of the outcome measures (21). When both soluble mediators and EV secretion were blocked by the cross-linking agent 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, the observed effects of hMSCs were fully abolished, whereas with the administration of mMSCs, they were partly ameliorated. These results demonstrated potent xenogeneic effects of CM and EVs in an immunocompetent mouse model of allergic airway inflammation (21).
A recent study assessed the effects of systemically administered adipose tissue-derived MSCs and their EVs. Both presented beneficial effects in ovalbumin-induced allergic asthma, acting on the inflammatory process and reversing tissue remodeling (52). While the effects of each were largely similar, differences were observed in outcome assessment of lung mechanics and inflammation: MSCs and EVs provoked different effects on eosinophil cell counts, levels of eotaxin, IL-4, and IL-13 in lung parenchyma, CD3+CD4+ T cells in BALF, and lung mechanics (52). This highlights the importance of in-depth studies of the differential mechanisms by which MSCs versus EVs might act in respiratory diseases.
ARDS
ARDS is a devastating condition that affects around 200,000 people each year in the United States alone, with mortality rates around 34.9–46.1% (75). Over the last decades, many novel therapeutic approaches have been tested for the treatment of ARDS, but none has proven efficient at targeting disease-specific pathways or reducing mortality; thus, supportive care measured, including antibiotics, protective mechanical ventilation strategies, and fluid restriction, remain the mainstays of therapy (76). With recent progress in the field of stem cells revealing their immunomodulatory, antibacterial, and regenerative properties (4), cell therapy has emerged as a potential candidate for ARDS treatment (77-79). Currently, eight clinical trials assessing the safety of cell therapy in patients with ARDS are registered in ClinicalTrials.gov (4-7); of these, several have demonstrated that administration of MSCs is not associated with adverse events (5-7), and one reported beneficial results when administered to two patients in a compassionate use setting (6).
Therapy with MSC-derived CM has already been tested and shown to lead to improvement of acute lung injury in mice (16,17). Additionally, EVs derived from human MSCs, when administered following E. coli endotoxin-induced acute lung injury in mice, reduced extravascular lung water by 43% and total protein levels in BALF by 35%, with a reduction in pulmonary edema and lung protein permeability (25). EVs also reduced neutrophil infiltration and macrophage inflammatory protein-2 levels in BALF by 73% and 49%, respectively, indicating a reduction in inflammation. Silencing KGF via siRNA pretreatment of MSCs partially abolished the therapeutic effects of the secreted EVs, suggesting that KGF played an important role in the underlying mechanism (25). In recent ARDS research, MSCs were shown to promote an anti-inflammatory and highly phagocytic macrophage phenotype through EV-mediated mitochondrial transfer. MSC-induced changes in macrophage phenotype depend critically on enhancement of macrophage oxidative phosphorylation. Furthermore, adoptive transfer of alveolar macrophages previously treated with MSC-derived EVs has been shown to reduce lung damage (43,44).
Pneumonia
Despite rapid advances in our armamentarium of antimicrobials, bacterial pneumonia is still associated with respiratory failure, and the case-fatality rate for this widely prevalent disease remains high in critically ill patients. In some settings, common treatment options may actually contribute to poor outcomes, as rapid lysis of pathogenic bacteria on the backdrop of an activated immune system may lead to inflammatory damage in the lung (80,81).
Therapy with MSCs has shown to be interesting due to its antimicrobial properties, as they are involved especially in dynamic coordination of the pro- and anti-inflammatory elements of the immune system or in increasing phagocyte activity, and directly by secretion of antimicrobial peptides and proteins (AMPs) (51). AMPs are evolutionarily conserved, gene-encoded small effector molecules that interact with different molecular targets either on the cell surface or within cells. Importantly, in some specific cases, AMPs can be active against pathogens that are resistant to conventional antibiotics (e.g., multidrug-resistant bacteria). In this context, MSCs from different sources or origins have shown ability to reduce the burden of pathogens in different preclinical models of pneumonia, regardless of the route, dose, or timing of administration (81,82).
Administration of human MSC-derived EVs decreased the influx of inflammatory cells, cytokines, protein, and bacterial load, resulting in higher survival rates of mice with bacterial pneumonia, in a mechanism partially dependent on keratinocyte growth factor secretion. The antimicrobial effect of BMSC-derived EVs was partly attributed to enhancement of monocyte phagocytosis of bacteria while decreasing inflammatory cytokine secretion, as well as to increased intracellular ATP levels in injured alveolar epithelial type 2 cells. The therapeutic effects of released EVs could be further enhanced by pre-stimulation of BMSCs with a TLR-3 agonist before isolation (53).
COPD
COPD, characterized by small-airway disease and parenchymal destruction, affects 5% of the global population and is the third leading cause of death worldwide, representing a substantial economic and social burden. COPD is inexorably progressive despite available pharmacologic treatments, which are mostly geared toward symptom relief (60). A growing number of investigations on MSC-based cell therapies for COPD are being conducted in experimental and clinical scenarios (8-10,54).
Recently, exosomes obtained from adipose-derived MSCs and artificial nanovesicles generated from the same cells were used in an elastase-induced emphysema model. Nanovesicles were generated by using sequential penetration through polycarbonate membranes, displayed a size (100 nm) and spherical shape resembling natural exosomes, and expressed both exosomal and stem-cell markers (54). Despite their beneficial effects, the disadvantage of exosomes for clinical applications is that they are only released naturally in very small amounts compared to nanovesicles (83). The proliferation rate of lung epithelial cells was increased in cells treated with MSC-derived artificial nanovesicles compared with cells treated with MSC-derived natural exosomes; a lower dose of MSC-derived artificial nanovesicles had similar regenerative capacity compared with a higher dose of MSCs and MSC-derived natural exosomes. Taken together, these data indicate that lower doses of ASC-derived artificial nanovesicles may have beneficial effects similar to those of higher doses of ASCs or ASC-derived natural exosomes in experimental emphysema, suggesting that artificial nanovesicles may have economic advantages that would warrant future clinical studies (54).
In parallel, the contribution of EVs derived from macrophages, epithelial cells, and endothelial cells to COPD pathophysiology highlights their potential as novel therapeutic targets. Elimination of these EVs, which contain nucleic acids or proteins as mediators of intracellular communication involved in disease pathogenesis, may be achieved through several different therapeutic approaches, including capture of circulating EVs, disruption of EV uptake by recipient cells, and inhibition of EV production or secretion (30,84).
Silicosis
Silicosis is the most common pneumoconiosis, with higher prevalence and incidence in developing countries. To date, there is no effective treatment to halt or reverse progression of the disease caused by silica-induced lung injury (2,11,85). Cell therapy has been tested in several studies and showed prominent effects, reducing lung fibrosis and promoting improvement in lung mechanics (2,85-88).
In this context, EVs derived from MSCs could reduce neutrophil and lymphocyte accumulation in BALF and reduce collagen deposition in lung parenchyma in silicotic mice (55,56). The same group showed that MSCs manage intracellular oxidative stress by targeting depolarized mitochondria to the plasma membrane via arrestin domain-containing protein 1-mediated microvesicles (56). The resulting vesicles are then engulfed and reutilized by macrophages, enhancing their bioenergetics. Furthermore, they have shown that MSCs simultaneously shed exosomes enriched with micro-RNAs that inhibit macrophage activation by suppressing Toll-like receptor signaling, thereby desensitizing macrophages to the ingested mitochondria. Collectively, these studies mechanistically link mitophagy and MSC survival with macrophage function, thus providing a physiologically relevant context for the innate immunomodulatory activity of MSCs both in vitro and in an in vivo model of lung injury (56).
IPF
IPF is a chronic, progressive, and inevitably fatal scarring lung disease, with a median survival as short as 3 years from the time of diagnosis, despite pharmacological therapies already approved by the U.S. Food and Drug Administration and in Europe (89). As such, the administration of MSCs is being investigated as a new therapeutic strategy for pulmonary fibrosis (90) in preclinical and clinical studies. MSCs can migrate to injured sites and secrete multiple paracrine factors, followed by regulation of endothelial and epithelial permeability, decrease of inflammation, enhancement of tissue repair, and inhibition of bacterial growth (90).
The recent discovery of therapeutic applications of EVs released from hMSCs has generated interest in their mechanisms of targeting and action. An in vivo efficacy study demonstrated that intravenous delivery of hMSC-EVs 14 days after induction of pulmonary fibrosis with intratracheal bleomycin significantly downregulated α-smooth muscle actin expression and decreased histopathological fibrosis, indicating therapeutic effects of these vesicles for established lung fibrosis through modification of the myofibroblastic phenotype (57).
PAH
PAH is a disease that mainly affects the pulmonary vascular bed. It is characterized by a proliferative disorder and resistance to apoptosis of the smooth muscle cells present in the pulmonary artery. This culminates in pulmonary artery remodeling and constriction, promoting an increase in pulmonary vascular resistance; this, in turn, is associated with compensatory hypertrophy of the right ventricle, which can rapidly progress to heart failure. The mean survival of untreated patients is 2.8 years after diagnosis, versus 3.6 years in treated patients (91).
Intravenous delivery of EVs derived from mouse MSC-CM suppressed influx of macrophages and the induction of proinflammatory and pro-proliferative mediators, including monocyte chemoattractant protein-1 and hypoxia-inducible mitogenic factor, in a murine model of hypoxic pulmonary hypertension. EVs also inhibited vascular remodeling and consequent pulmonary hypertension through suppression of the hypoxic activation of signal transducer and activator of transcription 3 (STAT3) and upregulation of the miR-17 superfamily of microRNA clusters, whereas it increased lung levels of miR-204, a key microRNA, expression of which is decreased in human pulmonary hypertension (58).
Recently, a group sought to determine which EV subpopulation plays a regulatory role in the reversal of PAH in mice. They found that the exosome fraction of EVs isolated from murine MSCs (MSC-EXOs) prevents and reverses PH in a monocrotaline-induced model of PAH. Furthermore, MSC-EXOs contain increased levels of miRNAs that blunt angiogenesis, inhibit proliferation of neoplastic cells, and induce senescence of vascular smooth muscle cells and endothelial progenitor cells. EXOs isolated from human MSCs were just as effective as those from murine MSCs in reversing pulmonary hypertension in mice (59). Together, these findings suggest a prominent role of EXOs in mediating the pulmonary vascular remodeling seen in PAH, and point to a promising therapeutic approach for its treatment (58,59).
Conclusions
Most studies analyzing the therapeutic effect of EVs have been performed in small-animal models and required only a small amount of EVs; therefore, large-scale manufacturing systems are needed to translate EV technology to the clinical trial setting. Furthermore, the same issue involved in isolating targeted EVs described below is still a limitation to clinical studies. In recent years, clear evidence of the involvement of EVs, especially exosomes and ectosomes, in the pathogenesis of lung diseases has emerged. The number and type of circulating EVs changes according as the natural history of lung diseases; the contents of EVs, such as microRNAs, are also changed by the disease condition. Therefore, EVs are promising candidates as novel biomarkers for lung diseases. EVs also act as a shuttle for transport of small molecules to distant cells, and modulate the function of the recipient cells. Due to this unique capability, EVs are also expected to have potential as a drug delivery system and as novel therapeutic targets. Research into EVs can provide new insights into the pathogenesis of various lung diseases and elucidate novel therapeutic approaches for respiratory medicine.
Acknowledgements
The authors would like to express their gratitude to Mrs. Moira Elizabeth Schöttler and Mr. Filippe Vasconcellos for their assistance in editing the manuscript.
Funding: This study was supported by the Carlos Chagas Filho Rio de Janeiro State Research Foundation (FAPERJ; grant number E-26/103.118/2014), Rio de Janeiro, Brazil; and the Brazilian Council for Scientific and Technological Development [CNPq; grant numbers 469716/2014-2, 465064/2014-0, 400462/2014-1, and 465656/2014-5 (INCT-REGENERA) to PRMR], Brasília, Brazil.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Weiss DJ. Concise review: current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells 2014;32:16-25. [Crossref] [PubMed]
- Lopes-Pacheco M, Bandeira E, Morales MM. Cell-Based Therapy for Silicosis. Stem Cells Int 2016;2016:5091838. [Crossref] [PubMed]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7. [Crossref] [PubMed]
- Cruz FF, Weiss DJ, Rocco PR. Prospects and progress in cell therapy for acute respiratory distress syndrome. Expert Opin Biol Ther 2016;16:1353-60. [Crossref] [PubMed]
- Zheng G, Huang L, Tong H, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res 2014;15:39. [Crossref] [PubMed]
- Simonson OE, Mougiakakos D, Heldring N, et al. In Vivo Effects of Mesenchymal Stromal Cells in Two Patients With Severe Acute Respiratory Distress Syndrome. Stem Cells Transl Med 2015;4:1199-213. [Crossref] [PubMed]
- Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med 2015;3:24-32. [Crossref] [PubMed]
- Weiss DJ, Casaburi R, Flannery R, et al. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest 2013;143:1590-8. [Crossref] [PubMed]
- Stolk J, Broekman W, Mauad T, et al. A phase I study for intravenous autologous mesenchymal stromal cell administration to patients with severe emphysema. QJM 2016;109:331-6. [Crossref] [PubMed]
- de Oliveira HG, Cruz FF, Antunes MA, et al. Combined Bone Marrow-Derived Mesenchymal Stromal Cell Therapy and One-Way Endobronchial Valve Placement in Patients with Pulmonary Emphysema: A Phase I Clinical Trial. Stem Cells Transl Med 2017;6:962-9. [Crossref] [PubMed]
- Morales MM, Souza SA, Loivos LP, et al. Pilot safety study of intrabronchial instillation of bone marrow-derived mononuclear cells in patients with silicosis. BMC Pulm Med 2015;15:66. [Crossref] [PubMed]
- Glassberg MK, Minkiewicz J, Toonkel RL, et al. Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest 2017;151:971-81. [Crossref] [PubMed]
- Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther 2016;7:125. [Crossref] [PubMed]
- Hansmann G, Fernandez-Gonzalez A, Aslam M, et al. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ 2012;2:170-81. [Crossref] [PubMed]
- Kim SY, Lee JH, Kim HJ, et al. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am J Physiol Lung Cell Mol Physiol 2012;302:L891-908. [Crossref] [PubMed]
- Ionescu L, Byrne RN, van Haaften T, et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol 2012;303:L967-77. [Crossref] [PubMed]
- Ionescu LI, Alphonse RS, Arizmendi N, et al. Airway delivery of soluble factors from plastic-adherent bone marrow cells prevents murine asthma. Am J Respir Cell Mol Biol 2012;46:207-16. [Crossref] [PubMed]
- Pierro M, Ionescu L, Montemurro T, et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax 2013;68:475-84. [Crossref] [PubMed]
- Sutsko RP, Young KC, Ribeiro A, et al. Long-term reparative effects of mesenchymal stem cell therapy following neonatal hyperoxia-induced lung injury. Pediatr Res 2013;73:46-53. [Crossref] [PubMed]
- Goolaerts A, Pellan-Randrianarison N, Larghero J, et al. Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am J Physiol Lung Cell Mol Physiol 2014;306:L975-85. [Crossref] [PubMed]
- Cruz FF, Borg ZD, Goodwin M, et al. Systemic Administration of Human Bone Marrow-Derived Mesenchymal Stromal Cell Extracellular Vesicles Ameliorates Aspergillus Hyphal Extract-Induced Allergic Airway Inflammation in Immunocompetent Mice. Stem Cells Transl Med 2015;4:1302-16. [Crossref] [PubMed]
- Abreu SC, Weiss DJ, Rocco PR. Extracellular vesicles derived from mesenchymal stromal cells: a therapeutic option in respiratory diseases? Stem Cell Res Ther 2016;7:53. [Crossref] [PubMed]
- Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012;126:2601-11. [Crossref] [PubMed]
- Zhang HC, Liu XB, Huang S, et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev 2012;21:3289-97. [Crossref] [PubMed]
- Zhu YG, Feng XM, Abbott J, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014;32:116-25. [Crossref] [PubMed]
- Sdrimas K, Kourembanas S. MSC microvesicles for the treatment of lung disease: a new paradigm for cell-free therapy. Antioxid Redox Signal 2014;21:1905-15. [Crossref] [PubMed]
- Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 2014;3:26913. [Crossref] [PubMed]
- Ragni E, Banfi F, Barilani M, et al. Extracellular Vesicle-Shuttled mRNA in Mesenchymal Stem Cell Communication. Stem Cells 2017;35:1093-105. [Crossref] [PubMed]
- Fujita Y, Yoshioka Y, Ito S, et al. Intercellular communication by extracellular vesicles and their microRNAs in asthma. Clin Ther 2014;36:873-81. [Crossref] [PubMed]
- Kadota T, Fujita Y, Yoshioka Y, et al. Extracellular Vesicles in Chronic Obstructive Pulmonary Disease. Int J Mol Sci 2016;17:E1801. [Crossref] [PubMed]
- Aliotta JM, Pereira M, Amaral A, et al. Induction of pulmonary hypertensive changes by extracellular vesicles from monocrotaline-treated mice. Cardiovasc Res 2013;100:354-62. [Crossref] [PubMed]
- Escudier B, Dorval T, Chaput N, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med 2005;3:10. [Crossref] [PubMed]
- Morse MA, Garst J, Osada T, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 2005;3:9. [Crossref] [PubMed]
- Besse B, Charrier M, Lapierre V, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2015;5:e1071008. [Crossref] [PubMed]
- Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014;28:970-3. [PubMed]
- Pachler K, Lener T, Streif D, et al. A Good Manufacturing Practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy 2017;19:458-72. [Crossref] [PubMed]
- Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014;14:195-208. [Crossref] [PubMed]
- Cantaluppi V, Gatti S, Medica D, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 2012;82:412-27. [Crossref] [PubMed]
- Mokarizadeh A, Delirezh N, Morshedi A, et al. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett 2012;147:47-54. [Crossref] [PubMed]
- Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 2013;10:301-12. [Crossref] [PubMed]
- Lo Sicco C, Reverberi D, Balbi C, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl Med 2017;6:1018-28. [Crossref] [PubMed]
- Song Y, Dou H, Li X, et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1beta-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells 2017;35:1208-21. [Crossref] [PubMed]
- Matthay MA. Extracellular Vesicle Transfer from Mesenchymal Stromal Cells Modulates Macrophage Function in Acute Lung Injury: Basic Science and Clinical Implications. Am J Respir Crit Care Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Del Fattore A, Luciano R, Pascucci L, et al. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant 2015;24:2615-27. [Crossref] [PubMed]
- Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 2009;20:1053-67. [Crossref] [PubMed]
- Bruno S, Grange C, Collino F, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 2012;7:e33115. [Crossref] [PubMed]
- Zhang B, Wang M, Gong A, et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015;33:2158-68. [Crossref] [PubMed]
- Shabbir A, Cox A, Rodriguez-Menocal L, et al. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev 2015;24:1635-47. [Crossref] [PubMed]
- Alcayaga-Miranda F, Gonzalez PL, Lopez-Verrilli A, et al. Prostate tumor-induced angiogenesis is blocked by exosomes derived from menstrual stem cells through the inhibition of reactive oxygen species. Oncotarget 2016;7:44462-77. [Crossref] [PubMed]
- Alcayaga-Miranda F, Cuenca J, Khoury M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front Immunol 2017;8:339. [Crossref] [PubMed]
- de Castro LL, Xisto DG, Kitoko JZ, et al. Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Res Ther 2017;8:151. [Crossref] [PubMed]
- Monsel A, Zhu YG, Gennai S, et al. Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am J Respir Crit Care Med 2015;192:324-36. [Crossref] [PubMed]
- Kim YS, Kim JY, Cho R, et al. Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp Mol Med 2017;49:e284. [Crossref] [PubMed]
- Choi M, Ban T, Rhim T. Therapeutic use of stem cell transplantation for cell replacement or cytoprotective effect of microvesicle released from mesenchymal stem cell. Mol Cells 2014;37:133-9. [Crossref] [PubMed]
- Phinney DG, Di Giuseppe M, Njah J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun 2015;6:8472. [Crossref] [PubMed]
- Shentu TP, Wong S, Espinoza C, et al. Extracellular vesicles isolated from human mesenchymal stem cells promote resolution of pulmonary fibrosis. FASEB J 2016;30:160.2.
- Aliotta JM, Pereira M, Wen S, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res 2016;110:319-30. [Crossref] [PubMed]
- Chen JY, An R, Liu ZJ, et al. Therapeutic effects of mesenchymal stem cell-derived microvesicles on pulmonary arterial hypertension in rats. Acta Pharmacol Sin 2014;35:1121-8. [Crossref] [PubMed]
- GOLD. GIfCOLD. Global Strategy for the Diagnosis Management and Prevention of COPD. 2017.
- Kupczyk M, Wenzel S. U.S. J Intern Med 2012;272:121-32. [Crossref] [PubMed]
- Bonfield TL, Koloze M, Lennon DP, et al. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol Lung Cell Mol Physiol 2010;299:L760-70. [Crossref] [PubMed]
- Park HK, Cho KS, Park HY, et al. Adipose-derived stromal cells inhibit allergic airway inflammation in mice. Stem Cells Dev 2010;19:1811-8. [Crossref] [PubMed]
- Nemeth K, Keane-Myers A, Brown JM, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A 2010;107:5652-7. [Crossref] [PubMed]
- Abreu SC, Antunes MA, de Castro JC, et al. Bone marrow-derived mononuclear cells vs. mesenchymal stromal cells in experimental allergic asthma. Respir Physiol Neurobiol 2013;187:190-8. [Crossref] [PubMed]
- Firinci F, Karaman M, Baran Y, et al. Mesenchymal stem cells ameliorate the histopathological changes in a murine model of chronic asthma. Int Immunopharmacol 2011;11:1120-6. [Crossref] [PubMed]
- Lee SH, Jang AS, Kwon JH, et al. Mesenchymal stem cell transfer suppresses airway remodeling in a toluene diisocyanate-induced murine asthma model. Allergy Asthma Immunol Res 2011;3:205-11. [Crossref] [PubMed]
- Ou-Yang HF, Huang Y, Hu XB, et al. Suppression of allergic airway inflammation in a mouse model of asthma by exogenous mesenchymal stem cells. Exp Biol Med (Maywood) 2011;236:1461-7. [Crossref] [PubMed]
- Goodwin M, Sueblinvong V, Eisenhauer P, et al. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells 2011;29:1137-48. [Crossref] [PubMed]
- Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy 2011;66:523-31. [Crossref] [PubMed]
- Lathrop MJ, Brooks EM, Bonenfant NR, et al. Mesenchymal stromal cells mediate Aspergillus hyphal extract-induced allergic airway inflammation by inhibition of the Th17 signaling pathway. Stem Cells Transl Med 2014;3:194-205. [Crossref] [PubMed]
- Cruz FF, Borg ZD, Goodwin M, et al. Freshly thawed and continuously cultured human bone marrow-derived mesenchymal stromal cells comparably ameliorate allergic airways inflammation in immunocompetent mice. Stem Cells Transl Med 2015;4:615-24. [Crossref] [PubMed]
- Cruz FF, Borg ZD, Goodwin M, et al. CD11b+ and Sca-1+ Cells Exert the Main Beneficial Effects of Systemically Administered Bone Marrow-Derived Mononuclear Cells in a Murine Model of Mixed Th2/Th17 Allergic Airway Inflammation. Stem Cells Transl Med 2016;5:488-99. [Crossref] [PubMed]
- Abreu SC, Antunes MA, Xisto DG, et al. Bone Marrow, Adipose, and Lung Tissue-Derived Murine Mesenchymal Stromal Cells Release Different Mediators and Differentially Affect Airway and Lung Parenchyma in Experimental Asthma. Stem Cells Transl Med 2017;6:1557-67. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Bein T, Grasso S, Moerer O, et al. The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Med 2016;42:699-711. [Crossref] [PubMed]
- Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med 2014;2:1016-26. [Crossref] [PubMed]
- Horie S, Masterson C, Devaney J, et al. Stem cell therapy for acute respiratory distress syndrome: a promising future? Curr Opin Crit Care 2016;22:14-20. [Crossref] [PubMed]
- Krasnodembskaya A, Samarani G, Song Y, et al. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol 2012;302:L1003-13. [Crossref] [PubMed]
- Finch S, Keir HR, Dicker AJ, et al. The past decade in bench research into pulmonary infectious diseases: What do clinicians need to know? Respirology 2017;22:1062-72. [Crossref] [PubMed]
- Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 2010;28:2229-38. [Crossref] [PubMed]
- Lee JW, Krasnodembskaya A, McKenna DH, et al. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med 2013;187:751-60. [Crossref] [PubMed]
- Théry C, Amigorena S, Raposo G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006;Chapter 3:Unit 3.22.
- Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles 2015;4:30087. [Crossref] [PubMed]
- Lopes-Pacheco M, Xisto DG, Ornellas FM, et al. Repeated administration of bone marrow-derived cells prevents disease progression in experimental silicosis. Cell Physiol Biochem 2013;32:1681-94. [Crossref] [PubMed]
- Lassance RM, Prota LF, Maron-Gutierrez T, et al. Intratracheal instillation of bone marrow-derived cell in an experimental model of silicosis. Respir Physiol Neurobiol 2009;169:227-33. [Crossref] [PubMed]
- Maron-Gutierrez T, Castiglione RC, Xisto DG, et al. Bone marrow-derived mononuclear cell therapy attenuates silica-induced lung fibrosis. Eur Respir J 2011;37:1217-25. [Crossref] [PubMed]
- Spitalieri P, Quitadamo MC, Orlandi A, et al. Rescue of murine silica-induced lung injury and fibrosis by human embryonic stem cells. Eur Respir J 2012;39:446-57. [Crossref] [PubMed]
- Ahluwalia N, Shea BS, Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med 2014;190:867-78. [Crossref] [PubMed]
- Li X, Yue S, Luo Z. Mesenchymal stem cells in idiopathic pulmonary fibrosis. Oncotarget 2015. [Epub ahead of print].
- Thenappan T, Shah SJ, Rich S, et al. A USA-based registry for pulmonary arterial hypertension: 1982-2006. Eur Respir J 2007;30:1103-10. [Crossref] [PubMed]
Cite this article as: Cruz FF, Rocco PR. Stem-cell extracellular vesicles and lung repair. Stem Cell Investig 2017;4:78.


