Extracellular vesicles as regulators of tumor fate: crosstalk among cancer stem cells, tumor cells and mesenchymal stem cells
Introduction
Tumors comprise several different cell types including tumor cells and surrounding non-tumorigenic cells such as fibroblasts together with immune, smooth muscle, and endothelial cells, collectively making up the so-called tumor microenvironment (1). Stem cell populations represent an important component of this microenvironment, regulating tumor growth, recruitment of immune cells, angiogenesis, invasion and metastasis (2,3). The interactions among tumor heterogeneous populations are mainly mediated by soluble molecules such as chemokines, cytokines, proteinases and growth factors (4). Recently, it has been suggested that extracellular vesicles (EVs) also provide a major mechanism of cell-to-cell communication in the tumor microenvironment (5). In this review we will focus on two stem cell populations, cancer stem cells (CSCs) and mesenchymal stem (stromal) cells (MSCs), which have mainly been studied in regard to their abilities to modulate the tumor microenvironment through secreting EVs.
EVs biology
EVs are a family of small lipid bilayer membrane vesicles commonly classified into three main groups: microvesicles, exosomes and apoptotic bodies. They differ in size, biogenesis and molecular content. They express specific receptors and lipids on their surfaces and encapsulate selected proteins and nucleic acids. EVs are released by almost all cell types and their content is a genetic representation of the cells from which they originate (6). Exosomes and microvesicles are usually released by living cells under physiological conditions. In contrast, apoptotic bodies, which will not be discussed in this review, are usually produced by cells undergoing an activated program of cell death.
Microvesicles (MVs, diameter range 100 nm–1 µm), also called membrane particles, are cell surface-derived EVs typically larger than exosomes and more heterogeneous in size. They originate from budding of the plasma membrane after specific stimuli (Figure 1). First described as released from activated blood platelets (7) and erythrocytes (8), they emerged as factors possibly involved in regulating the coagulation cascade (9). MV production is usually induced by increased levels of intracellular Ca2+, which mediates alterations in the distribution of phospholipids within the plasma membrane. The classical asymmetric lipid composition, also called the steady state, is characterized by the presence of certain phospholipids in the outside part of the cell membrane while the amino-phospholipids are concurrently maintained on the inner side (10). This balance is maintained by the membrane enzymes scramblase, flippase, floppase and translocase. Increased Ca2+ levels promote the transfer of phosphatidylserine from the inner part of the membrane and its exposure on the outer cell surface (11), this activity usually being associated with the recruitment and activation of the Ca2+-dependent enzyme scramblase (12) and cytoskeleton modification (7) via ATP-dependent mechanisms (13). In prostate and breast cancers, the ADP-ribosylation factor 6 (ARF6) involved in actin cytoskeleton remodeling and tumor cell invasion has been implicated in the shedding of all EV classes including MVs (14). For an extensive review regarding EVs, see (15). It should be noted that the so-called Large Oncosomes (LOs) are a specific class of EVs. Like MVs, LOs originate directly from plasma membrane budding (16), but they are larger, in the range 1–10 µm. It has been shown that LOs are released by highly metastatic prostate cancer (PCA) cells, since they are present in the blood of patients with metastatic disease (16), as revealed using the known PCA marker caveolin-1 (17).
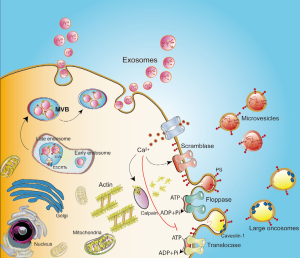
Exosomes are less variable in size, in the range 40–150 nm. They are stored as intraluminal vesicles (ILVs) within the endosome-derived multivesicular bodies (MVBs) and then released into the extracellular space after the MVBs fusion with the plasma membrane (18) (Figure 1). The mechanism of exosome packaging, membrane binding and sorting is complex and not yet completely elucidated. The formation of ILVs within the MVB involves the production of membrane curvature, inclusion of a specific cargo, and membrane separation for the release. The endosomal sorting complex required for transport (ESCRT) has been implicated in MVB and ILV biogenesis. ESCRTs consist of four different complexes (ESCRT-0, -I, -II and -III) associated with accessory proteins (ALIX, VPS4 and VTA1) and conserved during evolution (19). Proteins can be loaded in a ubiquitin-dependent manner into MVBs by the ESCRTs, where the ESCRT-0 and -I complexes are involved in capturing ubiquitinated membrane proteins (19) and the ESCRT-II and -III complexes are involved in membrane deformation processes and fission of ILVs (19,20). Interestingly, cells depleted of all four complexes can still produce CD63-positive EVs, indicating the existence of ILV loading systems independent of ESCRT (21).
The family of Rab GTPases seems key component in the coordination of vesicle traffic (22), including exosome release. Depending on the cell type, Rab11, Rab35 and/or Rab27 have been described as significant in exosome biogenesis and secretion (23). Interestingly, exosome release from mammary carcinoma cells lacking Rab27a is lower than from cells containing Rab27a, and tumor development is poorer, suggesting a pro-tumorigenic role for the exosomes (24). Janas et al. (25) identified different mechanisms involved in the sorting of RNA molecules contained in ILVs, mainly associated with a specific lipid bilayer binding motif and hydrophobic modifications in the RNA sequence. The concentration of the RNA molecules in the cytoplasm and the presence of raft-like regions enriched with specific lipids in the ILV-generating membrane seem to be crucial for RNA loading into ILVs (25).
EVs can transfer their content by activating specific signaling pathways in target cells. They can mediate cardinal biological processes related to tissue homeostasis (26), including stem cell maintenance and renewal (27), immune responses (28), and blood coagulation (29) among others. Otherwise, external stimuli or pathological states can modify the number and content of EVs (30,31), which nevertheless always resemble their cell of origin in both physiological and pathological conditions such as cancer (32). These characteristics and their isolation from body fluids such as urine (33), blood (34), cerebrospinal fluid (35), amniotic fluid and saliva (36) support their use as non-invasive biomarkers.
CSCs and their vesicles
CSCs, also defined as tumor-initiating cells, have been widely investigated in recent years. They are a small subpopulation of cancer cells that, according to CSC theory, contribute to tumor initiation and progression, metastasis formation, therapy resistance and cancer relapse (37). They share certain characteristics of normal stem cells such as a broad proliferation capacity, activation of common signaling pathways and expression of classical stem cell markers (38). However, in contrast to normal adult stem cells, which show precise differentiation and renewal properties, CSCs show multi-lineage differentiation capabilities and can generate different cancer subtypes (38).
CSCs have been isolated from many organs. Pioneering studies on acute myeloid leukemia (AML) identified a CSC population that expresses specific stem cell markers and exhibits stem cell properties (39). Moreover, these cells can reestablish human AML when transplanted into immunocompromised mice (39). CSCs were then identified by selective surface markers in multiple solid tumors such as those of the breast (40), brain (41), prostate (42), pancreas (43,44), colon (45,46) and kidney (47).
The origin of CSCs is still under debate. The earliest evidence, resumed in the so-called “hierarchical model”, led to the proposal that carcinogenesis is initiated by normal tissue stem cells that have been transformed by specific mutations and epigenetic alterations (48). The concept of cell reprogramming partly altered the notion of a static CSC population and characterized the CSCs as in a “continuum state”, in which mutated cancer cells can transiently switch through differentiated and stem cell-like phenotypes depending on external stimuli (49). Moreover, Mani et al. (50) demonstrated that the acquisition of tumor-initiating properties by mature or progenitor cancer cells during the de-differentiation process is mainly associated with the activation of specific pathways related to the epithelial-mesenchymal transition (EMT).
The tumor microenvironment has also been called the CSC niche (51) and it has been proposed as an important determinant for maintaining CSCs (52). Secretion of soluble factors by tumor-surrounding cells has been described as contributing to preserving CSCs in different tumors such as glioma (53), breast cancer (54) and pancreatic cancer (55). A bidirectional exchange of genetic information between CSCs and their niche is necessary to maintain the tumor (56), and EVs together with soluble factors have recently been implicated in CSC-niche interactions (Figure 2).
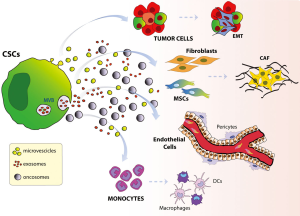
EVs in CSC niche
CSCs have been shown to release EVs that influence the surrounding niche (57). CSC-EVs can mediate direct crosstalk with other neoplastic cells or can modify normal surrounding cells to promote immune tumor escape, tumor growth and metastasis. Different studies have yielded evidence that EVs released by tissue-specific CSCs have a role in tumor progression, as reviewed in (57).
A small population of tumor-initiating cells called glioma stem cells (GSCs) has been reported to contribute to cancer initiation and propagation in glioblastomas (GBM) (58,59). Exosomes released from GSCs have been described as containing functionally active Cl− intracellular channel 1 protein (CLIC1), which appears to be directly involved in GBM proliferation both in vitro and in vivo (60). In addition, GSC-derived exosomes are efficiently internalized by CD14+ monocytes, which acquire a tumor-supportive phenotype by releasing pro-tumorigenic cytokines and could also contribute to T cell immunosuppression by stimulating tumor immune escape (61). In renal tumors, a CSC population expressing the CD105 marker release EVs able to activate angiogenesis and enhance lung metastasis, and characterization of their genetic content revealed groups of miRNAs and RNAs involved in these processes (62). Recently, it was observed that HLA-G expression in renal CSC-derived EVs is directly involved in the inhibition of monocyte differentiation into dendritic cells and therefore in tumor immune escape mechanisms (63).
Li et al. (64) demonstrated the ability of ductal carcinoma in situ (DCIS) stem-like cells to release exosomes containing lower levels of miR-140 and higher levels of miR-21 and miR-34 than exosomes derived from a whole DCIS cell population or normal stem cells. Interestingly, treatment with sulforaphane, an inhibitor of CSC proliferation (65), drastically modified the expression of these miRNAs in the DCIS stem-like cell exosomes, implying that the genetic content shuttled by EVs has a role in CSC signaling (66). In the same study, Kumar et al. (66) also demonstrated the ability of breast and prostate CSCs to release exosomes expressing the classical vesicle markers CD63, CD9, CD81, TSG101 and Alix without identifying selective surface molecules for those exosomes; however, anti-neoplastic treatments can modulate the levels of those markers, enhancing the release of exosomes from CSCs. Interestingly, when the miRNAs from exosomes released from prostate CSCs were compared with those contained in the EVs secreted by the cancer cells, the first population presented groups of differentially-expressed miRNAs associated with tumor progression and pre-metastatic niche formation, thus demonstrating how strongly the initiation of metastasis relies on the release of EVs from CSCs (67). In addition, exosomes released by PCA-initiating cells overexpressing CD44v6 (68) contribute to tumor progression by stimulating non-cancer initiating cells to acquire the CSC phenotype, complemented by the capacity of these exosomes to stimulate in vivo angiogenesis, invasion and host response (69).
The wide range of EV actions favoring tumor progression is also evidenced by other observations regarding their influence not only on tumor cells but also on different surrounding cells. Analyses of miRNA profiles in exosomes released by prostate CSCs versus non-CSC cells demonstrated enhanced EMT together with enrichment of metastasis-related miRNAs only in CSCs (70). Also interestingly, hypoxic prostate tumor cells can secrete exosomes, which are responsible for the enhanced stemness—increasing their ability to form spheroids—and invasiveness of “naïve” PCA cells by targeting adherent junction molecules (71). Moreover, they promote the acquisition of a cancer-associated fibroblast (CAF) phenotype by prostate fibroblasts (71), thus favoring angiogenesis, tumor growth due to increased nutrient acquisition, and metastasis. In the same way but in a different tissue, exosomes released by breast cancer cells overexpressing the CXCR4 stemness/metastatic molecule (72) increased primary tumor growth and metastatic potential in immunocompromised mice (73). These data further confirmed the ability of EVs released by cancer stem or stem-like cells to transfer their oncogenic features to recipient cells to promote cancer progression.
The CSCs can also be modified during interactions with surrounding cells. The CSC niche composed of non-tumorigenic cells can promote tumor survival by regulating CSC maintenance through EVs. Recently, Bourkoula et al. (74) demonstrated the potential of exosomes released by glioma-associated non-tumorigenic stem cells (GASC) to enhance the migration capacity and anchorage-independent growth of glioma CSCs. Moreover, Gernapudi et al. (75) showed that breast pre-adipocytes can enhance mammosphere formation by DCIS stem-like cells in vitro and tumor growth in vivo. Interestingly, mouse preadipocytes treated with shikonin, a natural anti-tumorigenic compound, generated exosomes unable to support CSC growth.
MSC vesicles and their key regulatory molecules
MSCs are present in specific niches of tissues such as bone marrow, adipose tissue, umbilical cord, and dental pulp from which they can easily be isolated and expanded in in vitro cultures (76). MSCs present self-renewal ability and multilineage differentiation into chondrocytes, osteocytes and adipocytes (mesoderm) and also into cells of ectodermic or endodermic origin (76). They have been widely investigated owing to their therapeutic potential in tissue injury or degenerative diseases, which is achieved through immunomodulatory regulation, angiogenesis, cell death inhibition, stimulation of proliferation and tropism from their niches (77-79). An important characteristic of these cells in supporting tissue recovery and regeneration is their chemotactic property, which accounts for their recruitment in response to injury and inflammation, with a tropism directed to injured sites. After engraftment into an injured site, MSCs interact with the surrounding cells not only by direct contact but also by paracrine actions (80).
The tumor microenvironment has some aspects similar to those observed in the inflammatory responses of an injured tissue owing to the secretion of molecules that promote MSC recruitment (81). Tumors release several chemokines and cytokines that have been shown to interact with MSC receptors. These include stromal cell-derived factor 1 (SDF-1), epidermal growth factor (EGF), platelet derived growth factor (PDGF), monocyte chemoattractant protein-1 (MCP-1), interleukin-8 (IL-8), IL-1β and tumor necrosis factor alpha (TNF-α), and have been shown to stimulate MSC tropism toward tumor niches (82). Although MSC mobilization to tumor niches has been well described (83), investigations into the role of these cells after engraftment have produced conflicting results. Several studies have reported a pro-tumorigenic role of MSCs by (I) inducing angiogenesis (84), drug resistance (85) and immune response escape (86), (II) stimulating EMT (87), and (III) promoting metastasis (88). On the other hand, an anti-tumorigenic effect of MSCs has been described: inhibition of tumor growth and inducing apoptosis (89,90). Such discrepancies could be attributable to the different protocols used, the origin of the MSCs and the tumor types, but the exact mechanisms involved are not well explained. However, the interaction between MSCs and tumor cells triggers specific responses in both cell types, mediated by direct and indirect interactions. Direct interactions result in the formation of shared nanotubes and gap junctions and also affect Notch signaling pathway, all associated with tumor invasion, therapy resistance, metastasis or tumor dormancy (83). The indirect interaction is characterized by secretion of groups of molecules such as TNF-α, IFN-γ and PGE2, which are related to the suppression of immune responses; and IL-6, IL-8, VEGF, PDGF and TGF-β associated with angiogenesis (83).
Another indirect but important mechanism in the interactions between MSCs and tumor cells is mediated by EVs. MSC-EVs constitute a heterogeneous population of exosomes, microvesicles and apoptotic bodies. MSCs secrete these vesicles constitutively, but alteration in the surrounding conditions such as hypoxia and chemical or physical stimuli can alter the amount of EVs secreted (91). As mentioned earlier, EVs contain proteins, lipids and genetic materials (mRNA and miRNAs) that have been thoroughly studied in large-scale analyses. The lipid moiety of MSC-EVs is enriched with diacylglycerol (DAG), sphingomyelin (SM) and ceramides, which are involved in signaling pathways and energy homeostasis (92). Ceramide is a known tumor suppressor that blocks the cell cycle and induces apoptosis. In addition, cancer cells can upregulate enzymes that redirect ceramide metabolism toward the promotion of mitogenicity, revealing a complex role of ceramide transfer by MSC-EVs in tumor fate (93). SM is a key component of lipid rafts, which are important signaling platforms (94), and changes of SM levels in the cell membrane can modify signaling pathways mediated by these microdomains. Reduction in SM levels has been associated with aggressive transformation of cancer cells, mediated by activation of oncoproteins and modifications of membrane composition (95). It should be emphasized that DAG mediates key signaling pathways by triggering several classes of proteins such as protein kinase C (PKC), which regulate different processes associated with proliferation, apoptosis, migration and tumorigenesis (96). Taken together, these data clearly demonstrate that bioactive lipids transferred by MSC-EVs from their membranes to the target cells can modulate different cellular processes involved in tumor physiology.
Besides lipids, MSC-EVs carry membrane proteins including surface markers that are used to characterize MSCs, such as CD13, CD29, CD44, CD73, CD90 and CD105. More importantly in the context of this review, MSC-EVs contain and transfer other proteins, the functions of which can be associated—among other options—with tumor physiology: TIMP-1, TIMP-2, MMP-9, MMP-2 (related to tumor growth and matrix remodeling) and VEGF and PDGF (related to angiogenesis) (92). Proteomic analysis of MSCs-derived EVs revealed a wide spectrum of proteins with biological functions associated with regulation of the cell cycle, proliferation, cell migration and angiogenesis (97,98). Collino et al. (99) analyzed the composition of MSC-EVs, showing differential protein enrichment among the subpopulations of EVs related to inflammation, interleukin, FGF and TGF-β pathways, which have implications for tumor evolution (100).
The other groups of molecules carried by MSC-EVs are the RNAs and miRNAs. Bone marrow-derived MSCs overexpress miR-23b, which induces a dormant state in a metastatic human breast cancer cell line through suppression of the target gene MARCKS, which encodes a protein involved in cell cycling and motility (101). Furthermore, miR-16 regulates VEGF expression and is also enriched in MSC-EVs. In vitro and in vivo experiments have shown that MSC-EVs promote the downregulation of VEGF mediated by transfer of miR-16, indicating that the EVs could have tumor suppressor activity (102). On the other hand, vesicular miR-21 was shown to stimulate renal cell carcinoma and breast carcinoma proliferation (92), revealing a pro-tumorigenic characteristic of MSC-EVs. These conflicting observations demonstrate that MSC-EVs have a dual role in tumor physiology. Different sources of MSCs and distinct tumor model studies are aspects well discussed by Klopp et al. (103) and could be responsible for this ambivalence.
Pro-tumorigenic role of MSC vesicles
Evidence of pro-tumorigenic activity by MSC-EVs has been reported in gastric and colon cancer cell line models that were injected into nude mice in the presence of EVs derived from MSCs, increasing tumor incidence and growth (104). The above-mentioned study by Vallabhaneni et al. (92) identified groups of lipids, proteins and RNAs with tumor supporting functions. In consequence, co-injection of MCF-7 breast cancer cells and MSC-EVs resulted in induction of tumor growth and angiogenesis. EVs secreted by MSCs originating from a different source, Wharton’s jelly, promoted renal carcinoma cell invasiveness and growth. The proposed molecular mechanism was MSC-EV-mediated transfer of HGF mRNA into the tumor cells, activating AKT and ERK1/2 signaling; these pathways are associated with tumor cell survival, growth and invasion, as demonstrated in (105).
Anti-tumorigenic role of MSC-derived vesicles
The anti-tumorigenic properties of MSCs also extend to their EVs, thus opening the possibility of using these vesicles in cancer treatment. Wharton’s jelly MSC-EVs can attenuate the growth of T24 bladder tumor cells in vitro and in vivo by arresting the cell cycle in G0/G1 phase and inducing cell death by apoptosis. Analysis of Akt/p-Akt by Western blotting revealed that the mechanism involved in the suppression of T24 proliferation is mediated by a reduction in phosphorylation but not in the total expression level of Akt (106). EVs derived from adipose MSCs also had tumor inhibiting effects. In vitro experiments showed that adipose MSC-EVs (I) reduce cell viability and wound-repair capacity and (II) inhibit the proliferation and colony-forming ability of A2780 ovarian cancer cells. Next-generation sequencing revealed several miRNAs including the miR-320, miR-127, miR-486, miR-423, miR-181, miR-423, miR-1246, miR-26, and miR-378 families, which have been linked to oncogene signaling pathways, and analyses of possible targets revealed that pro-apoptotic signaling molecules such as phosphorylated p53, BAX, activated CASP9, and activated CASP3 were upregulated (107).
EVs derived from bone marrow MSCs also show anti-tumorigenic properties in different tumor cell lines. Bruno et al. (108) examined the effects of human bone marrow MSC-EVs on three different tumor cell lines: HepG2 hepatoma, Kaposi’s sarcoma, and Skov-3 ovarian tumor cells. Tumor growth was inhibited in all cell lines co-injected with MSC-EVs in severe combined immunodeficiency mice, though the responses triggered in each cell were different. MSC-EVs induced cell death by apoptosis in HepG2 and Kaposi cells while Skov-3 cells underwent necrosis as the main cell death process. Gene array profiles also showed that different groups of genes related to cell cycle arrest were upregulated in each cell line after incubation with MSC-EVs. HepG2 hepatoma showed augmented expression of GTP-binding RAS-like 3 (DIRAS3), retinoblastoma-like 1 (Rbl-1), cyclin-dependent kinase inhibitor 2B transcript (CDKN2B), and cell cycle-negative regulator Rbl-2. In Kaposi cells, cyclin-dependent kinase inhibitor 1A transcript (CDKN1A), baculovirus IAP repeated-containing protein 5 (BIRC5), cyclin D1 (CCND1), and cell division cycle 20 homolog (CDC20) were upregulated, while the cyclin genes CCNE1 and CCND2 associated with the cell cycle G1/S transition were downregulated after EV incorporation. In contrast to HepG2 and Kaposi cells, Skov-3 cells presented no upregulated genes but only downregulated ones such as CCND2 (a regulatory subunit of CDK kinases) and CUL3 (a component of ubiquitin E3 ligase), which is correlated with mitotic division (108). Using the 4T1 breast cancer cell line, MSC-EVs were shown to inhibit angiogenesis in vivo and in vitro by inducing the downregulation of VEGF (102). In the latter study, the transfer of miR-16—carried by MSC-EVs—into tumor cells resulted in direct regulation of VEGF expression. Although the panel of anti-tumorigenic effects of EVs described in this review is far from complete, we can finally mention that those derived from a population of human liver stem cells—which express MSC markers—also shuttle groups of miRNAs such as miR-451, miR-223, miR-24, miR-125b, miR-31, and miR-122, which are associated with diminished drug resistance, cell cycle arrest and induction of apoptosis (109).
Factors associated with the dual role of MSC-EVs in tumors
Various studies cited in this review have presented copious evidence for the opposite actions of MSCs and MSC-EVs in tumors. This divergence could in part be attributed to different experimental models: in vitro/in vivo models, MSC origins, protocols for EVs isolation, and the amount and period of EV administration. In an attempt to understand the possible influence of MSC sources, Del Fattore et al. (110) studied the effects of EVs derived from bone marrow, umbilical cord and adipose tissue MSCs on glioblastoma cells. They found that EVs derived from bone marrow and umbilical cord inhibited tumor cell proliferation and induced apoptosis, while those derived from adipose tissue stimulated tumor proliferation, confirming the relevance of the cell source. The differences in mRNA and miRNA contents in the EVs derived from diverse MSCs (111) could underpin the different—and even opposite—regulatory responses by the recipient tumor cells.
Protocols of isolation can also determine the contradictory effects of EVs on tumors. Different procedures such as differential centrifugation, sucrose gradients, microfiltration, antibody-coated magnetic beads and microfluidic devices can isolate EV subpopulations (112) differently enriched with specific groups of proteins, mRNAs and miRNAs (99) that can trigger different responses in the tumor.
The actions of MSC-EVs on tumors can also depend of the recipient cell. Different tumor types and the time when EVs are administered can elicit different responses, even if the molecular information inside the vesicles is the same. This was observed in two separate studies by the same group examining the role of EVs from Wharton’’s jelly MSCs, resulting in the arrest of bladder cancer cell proliferation on the one hand (106) and the stimulation of growth and aggressiveness in renal cancer cells on the other (105). The tumor stage also seems to be an important aspect to consider in the effects of MSC-EVs: introducing MSCs into an established tumor inhibited tumor growth (113), whereas their administration during early tumor growth seemed to support tumor initiation (114). Since EVs are mediators of MSC action, the administration of MSC-EVs at different tumor stages can also trigger different responses.
Interactions between MSCs and tumor cells are not unidirectional. As previously discussed, tumor cells can modify the surrounding cells, inducing pro-tumorigenic phenotypes. This bidirectional crosstalk is partly mediated by EVs originating from both the MSCs and tumor cells, as shown by Yang et al. (115) (Figure 3). Peinado et al. (116) showed that tumor cells can modify bone marrow cells, promoting recruitment and “educating” them toward a pro-vasculogenic and pro-metastatic phenotype. These modifications in MSCs mediated by interaction with the tumor are associated with CAF formation and lead to recruitment of immune/inflammatory cells, enhancement of tumor malignancy, and metastasis (117). In a recent study, we showed that EVs secreted by renal CSCs induce a pro-tumorigenic phenotype in MSCs because of changes in their secretory profile. The EVs augmented the release of IL-8, which stimulated angiogenesis and tumor growth (118). Another aspect of this mutual interaction was observed in a mouse B16-F0 melanoma model, where tumor EVs confer on MSCs the capacity to promote macrophage infiltration into the tumor microenvironment through production of the CCR2 ligands, CCL2 and CCL7 (119). Although no studies have reported modifications in the vesicles secreted by MSCs after interactions with tumors, it is plausible that MSC-EVs can also be modified, changing from anti-tumorigenic to pro-tumorigenic characteristics.
Future perspectives
Discoveries regarding stem cell EVs in tumor communication within the niche have led to a new understanding of tumor physiology and opened a wide spectrum of possibilities in anti-tumorigenic therapies and clinical applications. The most tempting aspect of EVs is their capacity to encapsulate molecules such as proteins and nucleic acids, making them potential vehicles for delivery of anti-tumorigenic molecules. TNF-related apoptosis-inducing ligand (TRAIL) reportedly induces apoptosis preferentially in tumor cells, but administration of recombinant soluble TRAIL had limited efficacy (120). Yuan et al. (121) faced this challenge by infecting MSCs with a lentiviral vector encoding TRAIL: the cells started to produce EVs with high expression of TRAIL and administration of these EVs resulted in extensive death of different cancer cells. Using a different approach, Bronisz et al. (122) showed that miR-1 loading and delivery through EVs reduces tumorigenicity in stem-like glioblastoma cells and impairs EV-based microenvironmental communication; they proposed the use of EVs as therapeutic molecules for protein and miRNA delivery.
The use of EVs as suitable biomarkers for cancer diagnosis and prognosis without invasive processes has now emerged as a strong possibility. In fact, EVs represent a potential source of biomarkers for early detection and monitoring of tumor responses to treatment owing to their presence in almost all body fluids (123,124). Recently, Madhankumar et al. (125) targeted the interleukin 13 receptor α2 (IL13Rα2) on the surface of EVs derived from GSCs using ligand-conjugated quantum dots. Targeting specific markers makes possible the detection of tumor stem cells and exosomes in the serum or cerebrospinal fluid (CSF) of brain tumor patients by a non-invasive and simple diagnostic test (125). Clinical trials with EVs have already been approved and have shown good human tolerance to administration of the vesicles (126). At the same time, advances in gene therapy techniques such as suicide genes, silencing genes and miRNA-target genes to treat diverse genetic diseases (127) have created the possibility of combining the two strategies, resulting in encouraging prospects for cancer treatment.
Acknowledgements
The English style of this manuscript was corrected by BioMedES (UK), which is gratefully acknowledged.
Funding: This work was supported by Associazione Italiana per la Ricerca sul Cancro (A.I.R.C.) (Grant number IG12890), National Institute of Science and Technology for Regenerative Medicine REGENERA (Grant number 465656/2014-5); the Brazilian National Research Council (Grant numbers 404092/2012-8, 457222/2013-1, 403151/2015-5, 307605/2015-9); and the Carlos Filho Rio de Janeiro State Research Foundation (Grant numbers E-26/201.142/2014, E-26/010.001283/2015).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer 2007;7:139-47. [Crossref] [PubMed]
- Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015;16:225-38. [Crossref] [PubMed]
- El Marsafy S, Larghero J. Mesenchymal Stem Cells: Key Actors in Tumor Niche. Curr Stem Cell Res Ther 2015;10:523-9. [Crossref] [PubMed]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309-22. [Crossref] [PubMed]
- Roma-Rodrigues C, Fernandes AR, Baptista PV. Exosome in tumour microenvironment: overview of the crosstalk between normal and cancer cells. Biomed Res Int. 2014;2014:179486-96. [Crossref] [PubMed]
- Riazifar M, Pone EJ, Lötvall J, et al. Stem Cell Extracellular Vesicles: Extended Messages of Regeneration. Annu Rev Pharmacol Toxicol 2017;57:125-54. [Crossref] [PubMed]
- Fox JE, Austin CD, Boyles JK, et al. Role of the membrane skeleton in preventing the shedding of procoagulant-rich microvesicles from the platelet plasma membrane. J Cell Biol 1990;111:483-93. [Crossref] [PubMed]
- Fourcade O, Simon MF, Viode C, et al. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell 1995;80:919-27. [Crossref] [PubMed]
- Del Conde I, Shrimpton CN, Thiagarajan P, et al. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 2005;106:1604-11. [Crossref] [PubMed]
- Zwaal RF, Bevers EM. Platelet phospholipid asymmetry and its significance in hemostasis. Subcell Biochem 1983;9:299-334. [PubMed]
- Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev 2007;21:157-71. [Crossref] [PubMed]
- Dekkers DW, Comfurius P, Vuist WM, et al. Impaired Ca2+-induced tyrosine phosphorylation and defective lipid scrambling in erythrocytes from a patient with Scott syndrome: a study using an inhibitor for scramblase that mimics the defect in Scott syndrome. Blood 1998;91:2133-8. [PubMed]
- Beleznay Z, Zachowski A, Devaux PF, et al. ATP-dependent aminophospholipid translocation in erythrocyte vesicles: stoichiometry of transport. Biochemistry 1993;32:3146-52. [Crossref] [PubMed]
- Muralidharan-Chari V, Clancy J, Plou C, et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol 2009;19:1875-85. [Crossref] [PubMed]
- Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014;30:255-89. [Crossref] [PubMed]
- Di Vizio D, Morello M, Dudley AC, et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol 2012;181:1573-84. [Crossref] [PubMed]
- Tahir SA, Ren C, Timme TL, et al. Development of an immunoassay for serum caveolin-1: a novel biomarker for prostate cancer. Clin Cancer Res 2003;9:3653-9. [PubMed]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013;200:373-83. [Crossref] [PubMed]
- Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nat Rev Mol Cell Biol 2010;11:556-66. [Crossref] [PubMed]
- Chiaruttini N, Redondo-Morata L, Colom A, et al. Relaxation of loaded ESCRT-III spiral springs drives membrane deformation. Cell 2015;163:866-79. [Crossref] [PubMed]
- Stuffers S, Sem Wegner C, Stenmark H, et al. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009;10:925-37. [Crossref] [PubMed]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009;10:513-25. [Crossref] [PubMed]
- Blanc L, Vidal M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Bobrie A, Krumeich S, Reyal F, et al. Rab27a supports exosome-dependent and-independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 2012;72:4920-30. [Crossref] [PubMed]
- Janas T, Janas MM, Sapoń K, et al. Mechanisms of RNA loading into exosomes. FEBS Lett 2015;589:1391-8. [Crossref] [PubMed]
- Yáñez-Mó M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 2015;4:27066. [Crossref] [PubMed]
- Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006;20:847-56. [Crossref] [PubMed]
- Segura E, Nicco C, Lombard B, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naivevT-cell priming. Blood 2005;106:216-23. [Crossref] [PubMed]
- Than UT, Guanzon D, Leavesley D, et al. Association of Extracellular Membrane Vesicles with Cutaneous Wound Healing. Int J Mol Sci 2017;18:956-76. [Crossref] [PubMed]
- Lopatina T, Bruno S, Tetta C, et al. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal 2014;12:26-38. [Crossref] [PubMed]
- Riches A, Campbell E, Borger E, et al. Regulation of exosome release from mammary epithelial and breast cancer cells - a new regulatory pathway. Eur J Cancer 2014;50:1025-34. [Crossref] [PubMed]
- Melo SA, Sugimoto H, O'Connell JT, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014;26:707-21. [Crossref] [PubMed]
- Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 2004;101:13368-73. [Crossref] [PubMed]
- Caby MP, Lankar D, Vincendeau-Scherrer C, et al. Int Immunol 2005;17:879-87. [Crossref] [PubMed]
- Street JM, Barran PE, Mackay CL, et al. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med 2012;10:5-12. [Crossref] [PubMed]
- Keller S, Ridinger J, Rupp AK, et al. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med 2011;9:86-95. [Crossref] [PubMed]
- Islam F, Qiao B, Smith RA, et al. Cancer stem cell: fundamental experimental pathological concepts and updates. Exp Mol Pathol. 2015;98:184-91. [Crossref] [PubMed]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008;8:755-68. [Crossref] [PubMed]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730-7. [Crossref] [PubMed]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983. [Crossref] [PubMed]
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature 2004;432:396-401. [Crossref] [PubMed]
- Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005;65:10946-51. [Crossref] [PubMed]
- Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1:313-23. [Crossref] [PubMed]
- Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res 2007;67:1030-7. [Crossref] [PubMed]
- O'Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007;445:106-10. [Crossref] [PubMed]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007;445:111-5. [Crossref] [PubMed]
- Bussolati B, Bruno S, Grange C, et al. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J 2008;22:3696-705. [Crossref] [PubMed]
- Smalley M, Ashworth A. Stem cells and breast cancer: A field in transit. Nat Rev Cancer 2003;3:832-44. [Crossref] [PubMed]
- Scheel C, Eaton EN, Li SH, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 2011;145:926-40. [Crossref] [PubMed]
- Mani SA, Guo W, Liao MJ, et al. The epithelial mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704-15. [Crossref] [PubMed]
- Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell 2007;11:69-82. [Crossref] [PubMed]
- Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature 2011;474:318-26. [Crossref] [PubMed]
- Borovski T, Verhoeff JJ, ten Cate R, et al. Tumor microvasculature supports proliferation and expansion of glioma-propagating cells. Int J Cancer 2009;125:1222-30. [Crossref] [PubMed]
- Liu S, Ginestier C, Ou SJ, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res 2011;71:614-24. [Crossref] [PubMed]
- Lonardo E, Frias-Aldeguer J, Hermann PC, et al. Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle 2012;11:1282-90. [Crossref] [PubMed]
- Ye J, Wu D, Wu P, et al. The cancer stem cell niche: cross talk between cancer stem cells and their microenvironment. Tumour Biol 2014;35:3945-51. [Crossref] [PubMed]
- Hannafon BN, Ding WQ. Cancer stem cells and exosome signaling. Stem Cell Investig 2015;2:11-7. [PubMed]
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444:756-60. [Crossref] [PubMed]
- Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer 2006;6:425-36. [Crossref] [PubMed]
- Setti M, Osti D, Richichi C, et al. Extracellular vesicle-mediated transfer of CLIC1 protein is a novel mechanism for the regulation of glioblastoma growth. Oncotarget 2015;6:31413-27. [Crossref] [PubMed]
- Domenis R, Cesselli D, Toffoletto B, et al. Systemic T Cells Immunosuppression of Glioma Stem Cell-Derived Exosomes Is Mediated by Monocytic Myeloid-Derived Suppressor Cells. PLoS One 2017;12:e0169932. [Crossref] [PubMed]
- Grange C, Tapparo M, Tritta S, et al. Role of HLA-G and extracellular vesicles in renal cancer stem cell-induced inhibition of dendritic cell differentiation. BMC Cancer 2015;15:1009-18. [Crossref] [PubMed]
- Grange C, Tapparo M, Collino F, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res 2011;71:5346-56. [Crossref] [PubMed]
- Li Q, Eades G, Yao Y, et al. Characterization of a stem like subpopulation in basal like ductal carcinoma in situ (DCIS) lesions. J Biol Chem 2014;289:1303-12. [Crossref] [PubMed]
- Li Q, Yao Y, Eades G, et al. Downregulation of miR-140 promotes cancer stem cell formation in basal-like early stage breast cancer. Oncogene 2014;33:2589-600. [Crossref] [PubMed]
- Kumar D, Gupta D, Shankar S, et al. Biomolecular characterization of exosomes released from cancer stem cells: Possible implications for biomarker and treatment of cancer. Oncotarget. 2015;6:3280-91. [Crossref] [PubMed]
- Sánchez CA, Andahur EI, Valenzuela R, et al. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget 2016;7:3993-4008. [Crossref] [PubMed]
- Wang H, Rana S, Giese N, et al. Tspan8, CD44v6 and alpha6beta4 are biomarkers of migrating pancreatic cancer-initiating cells. Int J Cancer 2013;133:416-26. [Crossref] [PubMed]
- Wang Z, von Au A, Schnölzer M, et al. CD44v6-competent tumor exosomes promote motility, invasion and cancer-initiating cell marker expression in pancreatic and colorectal cancer cells. Oncotarget. 2016;7:55409-36. [Crossref] [PubMed]
- Rana S, Malinowska K, Zöller M. Exosomal tumor microRNA modulates organ cells. Neoplasia. 2013;15:281-95. [Crossref] [PubMed]
- Ramteke A, Ting H, Agarwal C, et al. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol Carcinog 2015;54:554-65. [Crossref] [PubMed]
- Ablett MP, O'Brien CS, Sims AH, et al. A differential role for CXCR4 in the regulation of normal versus malignant breast stem cell activity. Oncotarget 2014;5:599-612. [Crossref] [PubMed]
- Rodríguez M, Silva J, Herrera A, et al. Exosomes enriched in stemness/metastatic-related mRNAs promote oncogenic potential in breast cancer. Oncotarget 2015;6:40575-87. [Crossref] [PubMed]
- Bourkoula E, Mangoni D, Ius T, et al. Glioma-associated stem cells: A novel class of tumor-supporting cells able to predict prognosis of human low-grade gliomas. Stem Cells 2014;32:1239-53. [Crossref] [PubMed]
- Gernapudi R, Yao Y, Zhang Y, et al. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res Treat 2015;150:685-95. [Crossref] [PubMed]
- Rohban R, Pieber TR. Mesenchymal Stem and Progenitor Cells in Regeneration: Tissue Specificity and Regenerative Potential. Stem Cells Int 2017;2017:5173732. [Crossref] [PubMed]
- Peired AJ, Sisti A, Romagnani P. Mesenchymal Stem Cell-Based Therapy for Kidney Disease: A Review of Clinical Evidence. Stem Cells Int 2016;2016:4798639. [Crossref] [PubMed]
- Volarevic V, Nurkovic J, Arsenijevic N, et al. Concise review: Therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells. 2014;32:2818-23. [Crossref] [PubMed]
- Mora AL, Rojas M. Adult stem cells for chronic lung diseases. Respirology 2013;18:1041-6. [PubMed]
- Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther 2016;7:125. [Crossref] [PubMed]
- Spaeth E, Klopp A, Dembinski J, et al. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther 2008;15:730-8. [Crossref] [PubMed]
- Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 2007;25:1737-45. [Crossref] [PubMed]
- Melzer C, Yang Y, Hass R. Interaction of MSC with tumor cells. Cell Commun Signal 2016;14:20-32. [Crossref] [PubMed]
- Huang WH, Chang MC, Tsai KS, et al. Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene 2013;32:4343-54. [Crossref] [PubMed]
- Balakrishnan K, Burger JA, Quiroga MP, et al. Influence of bone marrow stromal microenvironment on forodesine-induced responses in CLL primary cells. Blood 2010;116:1083-91. [Crossref] [PubMed]
- Poggi A, Giuliani M. Mesenchymal Stromal Cells Can Regulate the Immune Response in the Tumor Microenvironment. Vaccines 2016;4:41-62. [Crossref] [PubMed]
- Mele V, Muraro MG, Calabrese D, et al. Mesenchymal stromal cells induce epithelial-to-mesenchymal transition in human colorectal cancer cells through the expression of surface-bound TGF-β. Int J Cancer 2014;134:2583-94. [Crossref] [PubMed]
- McAndrews KM, McGrail DJ, Ravikumar N, et al. Mesenchymal Stem Cells Induce Directional Migration of Invasive Breast Cancer Cells through TGF-β. Sci Rep 2015;5:16941-54. [Crossref] [PubMed]
- Zhu Y, Sun Z, Han Q, et al. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia 2009;23:925-33. [Crossref] [PubMed]
- Sun B, Roh KH, Park JR, et al. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy 2009;11:289-98. [Crossref] [PubMed]
- Ratajczak J, Wysoczynski M, Hayek F, et al. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 2006;20:1487-95. [Crossref] [PubMed]
- Vallabhaneni KC, Penfornis P, Dhule S, et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget 2015;6:4953-67. [Crossref] [PubMed]
- Morad SA, Cabot MC. Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer 2013;13:51-65. [Crossref] [PubMed]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000;1:31-9. [Crossref] [PubMed]
- de Almeida RF, Fedorov A, Prieto M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: Boundaries and composition of lipid rafts. Biophys J 2003;85:2406-16. [Crossref] [PubMed]
- Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer 2007;7:281-94. [Crossref] [PubMed]
- Anderson JD, Johansson HJ, Graham CS, et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells 2016;34:601-13. [Crossref] [PubMed]
- Kim HS, Choi DY, Yun SJ, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res 2012;11:839-49. [Crossref] [PubMed]
- Collino F, Pomatto M, Bruno S, et al. Exosome and Microvesicle-Enriched Fractions Isolated from Mesenchymal Stem Cells by Gradient Separation Showed Different Molecular Signatures and Functions on Renal Tubular Epithelial Cells. Stem Cell Rev 2017;13:226-43. [Crossref] [PubMed]
- Landskron G, De la Fuente M, Thuwajit P, et al. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014;2014:149185. [Crossref] [PubMed]
- Ono M, Kosaka N, Tominaga N, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 2014;7:ra63. [Crossref] [PubMed]
- Lee JK, Park SR, Jung BK, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One 2013;8:e84256. [Crossref] [PubMed]
- Klopp AH, Gupta A, Spaeth E, et al. Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells 2011;29:11-9. [Crossref] [PubMed]
- Zhu W, Huang L, Li Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett 2012;315:28-37. [Crossref] [PubMed]
- Du T, Ju G, Wu S, et al. Microvesicles derived from human Wharton's jelly mesenchymal stem cells promote human renal cancer cell growth and aggressiveness through induction of hepatocyte growth factor. PLoS One 2014;9:e96836. [Crossref] [PubMed]
- Wu S, Ju GQ, Du T, et al. Microvesicles derived from human umbilical cord Wharton's jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PLoS One 2013;8:e61366. [Crossref] [PubMed]
- Reza AM, Choi YJ, Yasuda H, et al. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci Rep 2016;6:38498. [Crossref] [PubMed]
- Bruno S, Collino F, Deregibus MC, et al. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev 2013;22:758-71. [Crossref] [PubMed]
- Fonsato V, Collino F, Herrera MB, et al. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells 2012;30:1985-98. [Crossref] [PubMed]
- Del Fattore A, Luciano R, Saracino R, et al. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin Biol Ther 2015;15:495-504. [Crossref] [PubMed]
- Collino F, Deregibus MC, Bruno S, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One 2010;5:e11803. [Crossref] [PubMed]
- Momen-Heravi F, Alian S, Mantel PY, et al. Current methods for the isolation of extracellular vesicles. Biol Chem 2013;394:1253-62. [Crossref] [PubMed]
- Otsu K, Das S, Houser SD, et al. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood 2009;113:4197-205. [Crossref] [PubMed]
- Beckermann BM, Kallifatidis G, Groth A, et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer 2008;99:622-31. [Crossref] [PubMed]
- Yang Y, Bucan V, Baehre H, et al. Acquisition of new tumor cell properties by MSC-derived exosomes. Int J Oncol 2015;47:244-52. [Crossref] [PubMed]
- Peinado H, Alečković M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012;18:883-91. [Crossref] [PubMed]
- Barcellos-de-Souza P, et al. Mesenchymal Stem Cells are Recruited and Activated into Carcinoma-Associated Fibroblasts by Prostate Cancer Microenvironment-Derived TGF-β1. Stem Cells 2016;34:2536-47. [Crossref] [PubMed]
- Lindoso RS, Collino F, Camussi G, et al. Extracellular vesicles derived from renal cancer stem cells induce a pro-tumorigenic phenotype in mesenchymal stromal cells. Oncotarget 2015;6:7959-69. [Crossref] [PubMed]
- Lin L Y, Du LM, Cao K, et al. Tumour cell-derived exosomes endow mesenchymal stromal cells with tumour-promotion capabilities. Oncogene 2016;35:6038-42. [Crossref] [PubMed]
- Carlo-Stella C, Lavazza C, Locatelli A, et al. Targeting TRAIL agonistic receptors for cancer therapy. Clin Cancer Res 2007;13:2313-7. [Crossref] [PubMed]
- Yuan Z, Kolluri KK, Sage EK, et al. Mesenchymal stromal cell delivery of full-length tumor necrosis factor-related apoptosis-inducing ligand is superior to soluble type for cancer therapy. Cytotherapy 2015;17:885-96. [Crossref] [PubMed]
- Bronisz A, Wang Y, Nowicki MO, et al. Extracellular vesicles modulate the glioblastoma microenvironment via a tumor suppression signaling network directed by miR-1. Cancer Res 2014;74:738-50. [Crossref] [PubMed]
- Katsuda T, Kosaka N, Ochiya T, et al. The roles of extracellular vesicles in cancer biology: toward the development of novel cancer biomarkers. Proteomics 2014;14:412-25. [Crossref] [PubMed]
- Kinoshita T, Yip KW, Spence T, et al. MicroRNAs in extracellular vesicles: potential cancer biomarkers. J Hum Genet 2017;62:67-74. [Crossref] [PubMed]
- Madhankumar AB, Mrowczynski O, Patel S, et al. Interleukin-13 conjugated quantum dots for identification of glioma initiating cells and their extracellular vesicles. Acta Biomater 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Mignot G, Roux S, Thery C, et al. Prospects for exosomes in immunotherapy of cancer. J Cell Mol Med 2006;10:376-88. [Crossref] [PubMed]
- Walther W, Schlag PM. Current status of gene therapy for cancer. Curr Opin Oncol 2013;25:659-64. [Crossref] [PubMed]
Cite this article as: Lindoso RS, Collino F, Vieyra A. Extracellular vesicles as regulators of tumor fate: crosstalk among cancer stem cells, tumor cells and mesenchymal stem cells. Stem Cell Investig 2017;4:75.


