TLR4 in glioblastoma—when cancer stem cells ignore “danger signals”
The microenvironment of glioblastoma is hostile to both invading and resident cells. Tumor cells in the tumor core are challenged by hypoxia, acidosis and have to resist attacks of invading microglia cells and other invading immune cells (1,2). In their recent paper, Alvarado et al. studied the role of Toll-like receptors (TLR) in glioblastoma cancer stem cells (CSC) and tumor cells without stem cell properties (3). TLRs have a well-established role in the innate immune system: Endogenous molecules liberated from damaged tissue or by pathogen-associated molecular patterns (e.g., lipopolysaccharides) activate TLR receptors during tissue injury or infection (4). This allows the detection of damage and of pathogen associated molecular pattern (“danger signals”) and constitutes a crucial mechanism for activation of the innate immune system and especially dendritic cells.
Why should one study the expression and function of TLRs in glioblastoma cells? In recent years, it has become more and more clear that TLRs have functions beyond the immune system (4). TLRs are expressed on all cells in the CNS and not only microglia. In embryonal neural stem cells, TLR signaling also modulates stemness clearly proving that TLR signaling goes beyond the detection of “danger” or “damage” by the innate immune system (5). However, what would the role of TLRs in glioblastoma be? One putative function may be promotion of tumor cell growth. It is not uncommon that tumor cells use physiological signaling pathways. Moreover, strategic mutations may turn anti-oncogenic into oncogenic and growth promoting signaling pathways favoring the tumor growth. The role of TGF-beta signaling in breast cancer progression may be a good example of this mechanism (6).
The role of TLRs in glioblastoma turned out to be completely different (3). Alvarado et al. focused on TLR4, which was one of the three TLRs with a pronounced differential expression in CSC and the most pronounced differential response to TLR stimulation. Little was known on the function of TLR4 on glioma cells before this study. Just one histological study by Lin et al. proposed that TLR4 is upregulated in human glioblastoma (7).
Alvarado et al. confirmed this result but found that TLR4 expression is absent in human glioblastoma CSC. In contrast, more differentiated glioblastoma cells expressed TLR4. A series of elegant experiments then unveiled the reason for this differential expression: TLR4 expression and signaling substantially suppressed CSC properties contributed to the non-stem cell phenotype. The expression of TLR4 was the crucial difference between CSC and non-CSC. The surprising new finding in this publication is that the signaling cascade downstream of TLR4 remained functionally active despite of TLR4 downregulation. The key proteins TBK1 and the transcription factor RBBP5 are expressed in glioblastoma cells and especially in CSC.
This finding suddenly makes the lack of TLR4 signaling interesting. Activation of TLR4 signaling pathway, e.g., via RBBP5 inhibition, may be a very attractive and new therapeutic target. It would allow suppressing stem cell properties in CSC (Figure 1) and may result in a substantial inhibition of tumor growth. Alvarado et al. tested this hypothesis in an orthotropic murine model. Inhibition of RBBP5 expression using shRNA in CSC resulted in a highly significant improvement of survival in mouse as compared to control CSC. This clearly indicates and substantiates the potential therapeutic relevance of targeting the TLR4 signaling pathway.
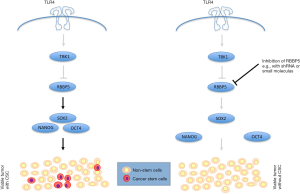
Of course, the way from a single series of experiments to the cure of patients is long and a couple questions remained unaddressed. At the moment, it remains unknown, if RBBP5 expression is relevant in glioblastoma and if RBBP5 is expressed in all tumors. A screen in the Repository for Molecular BRA in Neoplasia DaTa (REMBRANDT) database (www.Betastasis.com) showed a very variable expression of RBBP5 in gliomas of different grade. It indicates that this signaling pathway may not be relevant for all gliomas and this may become an issue when testing this new and interesting signaling pathway in a larger series of glioblastoma. Detailed and high quality histological studies are certainly needed. Another unknown question remains whether activation of TLR4 signaling by RBBP5 inactivation may have unwanted systemic side effects, e.g., in the innate immune system.
Irrespective if future experiments will successfully resolve the these obvious and typical challenges of any new promising molecular therapeutic or not: Alvardo et al. found a completely new and previously not know signaling pathway in glioblastoma CSC, that gives new insights in the function of TLR4 far beyond the immune system.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol 2004;14:198-206. [Crossref] [PubMed]
- Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer 2007;7:733-6. [Crossref] [PubMed]
- Alvarado AG, Thiagarajan PS, Mulkearns-Hubert EE, et al. Glioblastoma Cancer Stem Cells Evade Innate Immune Suppression of Self-Renewal through Reduced TLR4 Expression. Cell Stem Cell 2017;20:450-61.e4. [Crossref] [PubMed]
- Hanke Mark L, Kielian T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clinical Science 2011;121:367-87. [Crossref] [PubMed]
- Rolls A, Shechter R, London A, et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol 2007;9:1081-8. [Crossref] [PubMed]
- Massagué J. TGFβ in Cancer. Cell 2008;134:215-30. [Crossref] [PubMed]
- Lin CK, Ting CC, Tsai WC, et al. A tissue microarray study of toll-like receptor 4, decoy receptor 3, and external signal regulated kinase 1/2 expressions in astrocytoma. Indian J Pathol Microbiol 2016;59:294-300. [Crossref] [PubMed]
Cite this article as: Beier CP, Kristensen BW. TLR4 in glioblastoma—when cancer stem cells ignore “danger signals”. Stem Cell Investig 2017;4:66.


