Strategies for retinal cell generation from human pluripotent stem cells
Introduction
Shinya Yamanaka’s 2006 discovery that specialized adult cells could be reprogrammed into a stem cell-like state revolutionized our ability to study and develop disease-specific models (1). Induced pluripotent stem cells (iPSCs) are specialized self-renewing cells that are generated by exogenously expressing pluripotency-associated transcription factors in somatic cells such as fibroblasts, peripheral blood mononuclear cells, or lymphoblastoid cell lines (LCLs). Prior to this discovery, the only known naturally pluripotent cells were embryonic stem cells (ESCs). Ethical concerns regarding the origins of human ESCs (hESCs) made research and any subsequent applications of the cells challenging. iPSCs are functionally similar to hESCs in their capacity for indefinite self-renewal and potential to differentiate into all human cell types, eliminating the ethical burden and allowing for prior developmental assays to be applied across any genetic background interchangeably (2-6). Mouse and human pluripotent stem cells have been used for a variety of applications such as studying embryonic development, disease modeling, drug screening, and cell replacement strategies. iPSCs offer a personalized approach to pathological studies, and are particularly useful for diseases that lack appropriate animal models.
Mutations causing hereditary retinal degenerative diseases have been identified in >265 genes and affect all human retinal cell lineages (7). It is impossible to have an animal model for each affected gene, let alone all the possible mutations of a gene. iPSC technology provides a unique opportunity to generate personalized developmental and disease models by reprogramming a patient’s skin or blood cells and differentiating them into the retinal cell of interest using one of many differentiation strategies first established using mouse or human ESCs. Translating these protocols for human iPSC (hiPSC) differentiation was essential for developing gene and autologous cell replacement therapies. This review will describe the strategies to generate retinal pigment epithelium (RPE), neural retinal progenitors and precursors, and mature neural retinal cells using monolayers or three-dimensional cultures of pluripotent stem cells.
In vivo eye development
Organogenesis of the vertebrate eye begins with a pair of evaginations of the neuroepithelium from the neural tube mediated by the retinal homeobox transcription factor, RAX (8-11). These diverticula extend from the diencephalon and form hollow bulbs known as the optic vesicles. At this stage, the progenitors of the neuroepithelium have the potential to differentiate into the inner neural retina or the outer RPE. These cells are characterized by the expression of RAX as well as other key regulators of eye field specification, PAX6, LHX2, SIX3, and SIX6 (12). Ubiquitous expression of the microphthalmia transcription factor (MITF) regulated by OTX2 in the optic vesicle controls the bipotentiality of the neuroepithelium (13,14). The subsequent downregulation of these two transcription factors by VSX2 in the distal region and extrinsic signaling of fibroblast growth factors (FGF) from the overlying ectoderm initiate domain specification and define the population of inner retinal progenitor cells (RPCs) (15-18). Presumptive RPE will continue to express MITF.
Interactions with the surface ectoderm and extraocular mesenchyme initiate a thickening of the ectoderm into the lens placodes, which cause an axial invagination of the distal portion of the optic vesicles to form the optic cups. These structures consist of an RPE and neural retinal progenitor bilayer of cells. RPCs then proliferate to form a stratified structure, eventually giving rise to lineage-restricted precursor cells that will mature into postmitotic retinal neurons and Müller glia. Table 1 provides a list of retinal cell markers for each of these stages of development.
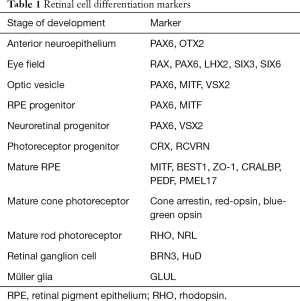
Full table
RPE differentiations
RPE is the most well-characterized retinal cell differentiation. The RPE is a monolayer of cells that structurally and metabolically supports the underlying neurosensory retina, including the photoreceptors, and forms part of the blood-retinal barrier. It functions mainly in nutrient and ion transport, phagocytosis of shed photoreceptor outer segments, light absorption, and vitamin A metabolism (19-23). Dysfunction of this specialized tissue can lead to photoreceptor dystrophy and blindness, such as that associated with age-related macular degeneration (AMD), the leading cause of irreversible blindness in the maturing population worldwide (24). RPE cells are most easily identified by their hexagonal geometry and pigmentation.
For more than a decade, RPE have been generated from pluripotent stem cells by spontaneous differentiate upon removal of basic fibroblast growth factor (bFGF) from the cell culture medium (25-32). Gene expression profiling asserted the quantitative similarity between RPE generated using this strategy and human fetal RPE. Initial pigmentation typically appeared 25-30 days after the start of differentiation, although this was cell-line dependent and can vary considerably between differentiations. Foci of pigmented cells large enough for dissection and enrichment occurred 60–90 days after bFGF depletion. These populations of cells have RPE hexagonal geometry, pigmentation, stain for classic protein markers such as Best1, ZO-1, MITF, and PEDF, have phagocytosis potential, and generate a transepithelial resistance (TER) comparable to fetal-derived RPEs. However, only after eight months in culture do cells start to express RPE65, a hallmark of terminally differentiated RPE.
The next attempt to increase differentiation kinetics of RPE involved the addition of nicotinamide (NIC) and Activin A to pluripotent cells (33-36). Table 2 describes the function of these small molecules and others in retinal cell differentiations. Large numbers of pigmented clusters could be identified after only six weeks, significantly increasing RPE generation dynamics. Additional factors were introduced as retinal-inducing factors: noggin, retinoic acid, and sonic hedgehog (37-44). RPE generation was significantly increased when using MITF positive+ cells as a readout (37). More recently, emulating in vivo RPE development cues in vitro proved useful for rapid RPE-directed differentiation (45-47). Combined use of insulin-like growth factor 1 (IGF1), noggin, bFGF, and dickkopf-related protein 1 (Dkk1) with the serial application of NIC, Activin A, fibroblast growth factor receptor 1 (FGFR1) inhibitor SU5402, and vasoactive intestinal peptide (VIP) allowed for ~80% RPE generation efficiency with hESCs and ~60% efficiency with hiPSCs based on PMEL17 expression (45). Gene expression analysis revealed an accelerated ocular morphogenesis from early eye field to optic vesicle and RPE when compared to in vivo development. Sheets of RPE expressing critical RPE markers could be obtained in as little as 14 days and homogenous cultures of RPE produced in three weeks.

Full table
Maruotti et al. described an alternative approach to hiPSC-RPE differentiation utilizing small molecules which are favored for potential clinical applications over growth factors derived from animal or bacterial cells, which are liable to vary from lot-to-lot and escalate the potential for patient infection or immune rejection. Small-molecule chetomin (CTM) was identified in a high-throughput qPCR screen to consistently upregulate RPE markers MITF, OTX2, and PMEL17 (48). CTM is a metabolite of the fungus Chaetomium species that inhibits the transcriptional activation of the hypoxia-inducible factor (HIF) pathway (49). In combination with NIC, CTM potently induces directed-RPE differentiation in multiple hESC and hiPSC lines as measured by marker expression. Typical RPE morphology is achieved by switching hESC medium to RPEM two weeks post cotreatment with NIC and CTM. Pure monolayers of functional RPE can be obtained following a single passage of these cultures.
Although many recent publications highlight the advances in RPE differentiation protocols, substantial sources of variability in the efficiency of RPE generation include cell line, genetic background, passaging method, passage number, seeding density, and extracellular matrix (50). All of these models have been valuable for the generation of in vitro-derived RPE that have been used for phase I clinical trials for the treatment of macular degeneration (51). Reproducible differentiations are paramount for the clinical success of hiPSC-RPE based therapies.
Adherent neural retinal differentiations
The RPCs of the neuroblastic layer of the optic cup divide symmetrically during early development to increase the progenitor pool size in preparation to give rise to the seven different mature cell types of the neural retina – retinal ganglion cells, amacrine cells, bipolar cells, horizontal cells, rod photoreceptors, cone photoreceptors, and Müller glia (52). This process is coordinated in time and space by both intrinsic and extrinsic factors (53). Eventually the progenitor pool begins to divide asymmetrically to generate daughter cells that can adopt differing fates during development (54). Differentiation into specific post-mitotic precursor cells is initiated upon termination of proliferation. These precursors then migrate towards the prospective layer of the neural retina in which the mature cell will reside (55). Multipotent RPCs and the precursors that they give rise to are of particular interest for therapeutics due to the number of degenerative conditions the neural retina is subject to.
Inherited retinopathies, such as retinitis pigmentosa (RP), Leber congenital amaurosis (LCA), and Stargardt disease (STGD), are a genetically and phenotypically heterogeneous group of monogenic diseases that mainly effect genes expressed in the light-sensitive rod and cone photoreceptors. Mutations in more than 250 identified genes can affect almost every aspect of photoreceptor function causing diverse pathophysiologies that culminate in cell death (56). Alternative pathogenic variants of the same gene can cause multiple disparate retinal diseases (7). This complex etiology necessitates the need for iPSC technology for multiple reasons. Often an affected gene or specific mutation will lack a corresponding animal model, making pre-clinical treatment studies difficult or impossible to extrapolate to humans. hiPSCs provide a unique opportunity to generate personalized models of disease to be used in a variety of studies, from determining pathology to demonstrating intervention efficacy (57). Additionally, they are a source of autologous cells for transplantation. The generation of RPCs and further retinal lineages has been performed using either adherent cell aggregations into spheroid structures or densely packed monolayer cultures.
In vitro differentiation to RPCs and photoreceptor precursors requires several sequential steps beginning with the induction of an anterior neural fate by inhibition of Wingless (Wnt) and bone morphogenic protein (BMP) (58-62). Three week culturing of adherent cell aggregates, known as embryoid bodies (EBs), of the H-1 hESC line with potent Wnt and BMP inhibitors Dkk1 and noggin, respectively, IGF-1, and bFGF exhibit increased expression of eye field transcription factors (EFTFs) and retinal progenitor markers in comparison to undifferentiated and factor-free cultures (63). Immunofluorescence labeling with specific retinal neuron antibodies revealed a preference for ganglion and amacrine precursor cells. While markers for immature photoreceptors, CRX and NRL, were also expressed in approximately 12% of cells, very few mature photoreceptor markers were present in the cultures. Similar results were obtained with hiPSC using this method (64).
Based on differentiations with mouse and monkey ESCs, initial treatment of EBs of the khES-1 hESC line with Dkk1 and Nodal antagonist, LeftyA, resulted in approximately 16% RX+/Pax6+ colonies after 35 days in culture (65). Nodal is a member of the transforming growth factor β superfamily. Upon addition of retinoic acid and taurine, factors that can promote the terminal differentiation of photoreceptors, CRX+ cells began to appear in culture at 90 days and increased as the culture period was extended to 120 (~11% of total cells) and 170 (~20% of total cells) days. Rhodopsin (RHO)+ rod photoreceptors were present by day 130 and also increased in number to ~8.5% of total cells by day 200. Red/green opsin (OPN1LW)+ or blue opsin (OPN1SW)+ cells could also be found in close proximity to RHO+ cells. The results were replicated with the khES-3 hESC line. These putative photoreceptors expressed additional genes involved in phototransduction. Although time-consuming and inefficient, this study used a chemically defined culture medium which has better potential for clinical application. Later differentiations utilizing nonbiological small-molecules Y-27632, CKI-7, and SB-431542 to inhibit Wnt and Nodal signaling made greater strides towards xeno-free culture systems, but yielded a lower percentage of RHO+ cells than cultures treated with Dkk1 and LeftyA (66).
Mellough et al. combined some aspects of these two previous studies with additional modifications to mimic neural and retinal development in an attempt to design a photoreceptor differentiation protocol for hESC and hiPSC with higher yields and less culture time (67). The three-step differentiation resulted in 16% of CRX+ cells and the appearance of late-stage photoreceptor-specific markers RHO, OPN1LW, and OPN1SW by day 45, representing a dramatic decrease in time to differentiation from previous reports (65). However, the population of photoreceptor marker positive cells rapidly declined by day 60. Interestingly, control populations differentiated under basal media conditions supplemented only with B27 supplement and, at later time points, B27 and N2 supplements, exhibited neuronal, eye field, retinal, and photoreceptor gene expression, revealing the importance of B27 and N2 in serum-free media conditions for photoreceptor generation within the shortened culturing time frame. Both B27 and N2 are chemically defined blood serum substitutes consisting of multiple hormones, including insulin and progesterone, and other growth factors that promote neuronal cell survival (68-71).
To date, the protocol that yields that highest amount of photoreceptors from hESCs in the shortest amount of time utilizes a human recombinant protein known as COCO, a multifunctional product of the Cerberus gene family expressed in the developing and adult mouse retina that inhibits BMP, TGFβ, and Wnt signaling pathways (72). COCO has also been shown to antagonize other ligands of the TGF-beta superfamily (which includes BMP and TGFβ) with roles in retinal development, Nodal and Activin (73,74). In combination with bFGF and IGF1 treatment, the H9 hESC line differentiated to induce expression of early retinal and photoreceptor genes SIX6 and CRX and phototransduction gene OPN1SW ~10-fold more effectively than with noggin and Dkk1 instead of COCO after 21 days of culture (75). S-opsin was expressed by ~70% of differentiated cells at this time point. The additional blocking of TGFβ signaling appears to be paramount to the success of this protocol as compared to previous methods that inhibit only BMP and Wnt or Wnt and Nodal signaling (63-67). These findings also suggest that antagonizing all of these signaling pathways is required for cone genesis at the expense of rod genesis, which measured by marker expression appeared to be the more prevalent photoreceptor in the retinal cultures of the previously established protocols.
Three-dimensional organoids
Perhaps the most relevant model for studying inherited retinal disease pathologies and their potential treatments is the 3D-stratified retinal organoids first developed with mouse ESCs and later recapitulated with hESCs (76-79). hESCs dissociated into single cells are reaggregated in low-cell-adhesion V-bottom 96-well plates at a density of 9,000–12,500 cells/well in retinal defined differentiation medium containing ROCK inhibitor (Y-27632) and Wnt inhibitor (iWR1e). Matrigel is added to the culture medium shortly following initial plating as a basement membrane for structural development of epithelial character. Addition of fetal bovine serum (FBS) and hedgehog signaling pathway smoothened agonist (SAG) culminate in more than 70% of total cells expressing early retinal marker, RAX (77). These conditions favor the formation of VSX2+/PAX6+ neural retina fated cells, but treatment with Wnt agonist CHIR99021 during days 18-21, after the cells have committed to a retinal fate, could efficiently induce swaths of MITF+ presumptive RPE without disturbing VSX2 expression in the distal portion of the retinal epithelium to be more representative of developing optic cups in vivo.
The autonomous appearance of evaginations reminiscent of optic vesicles and their subsequent invagination to form optic cups from homogenous hESC aggregates observed in these protocols appears to closely mimic the complex tissue interactions of in vivo retinogenesis (80). However, these structures develop at low efficiency and their successful dissection is what determines the retinal organoid yield, which can be quite limited. Emitting dissection risks compromising the retinal identity of organoids with integration of non-retinal structures (81,82). Völkner et al. provide a protocol for unbiased neuroepithelium trisection at the eyefield stage to produce high numbers of large, stratified organoids committed to retinal fate (79). Another modification to the Nakano et al. protocol that may increase the differentiation efficiency to retinal progenitors and neural retina within individual organoids is the lowering of oxygen tension from atmospheric 20% to 2%, which is more representative of the physiologic oxygen level during human organogenesis. After 21 days in a 2-dimensional culture system, a greater number of hiPSC-RPCs and hESC-RPCs were PAX6+/VSX2+ in 2% oxygen compared to 20% oxygen (83). qPCR analysis also revealed elevated expression of PAX6 and VSX2 in RPCs kept at 2% oxygen. In contrast, optic vesicles are maintained in 40% oxygen long-term (77,79). In vitro differentiation efficiency may benefit from replicating in vivo conditions, but the ability to preserve multilayered organoids without a vascular supply of nutrients requires higher oxygen tension and organoid agitation (84).
Maturation of hESC-derived optic cups over several weeks (day 47–120) leads to the differentiation of CRX+/RECOVERIN+ photoreceptors (79). This process can be accelerated with inhibition of Notch signaling by DAPT (76,77,79,85). However, DAPT treatment appears to favor cone photoreceptor genesis over rod photoreceptor genesis, consistent with reports of Notch suppression of cone fate specification (86,87). The presence of retinal ganglion and amacrine cells can be detected by day 37, while horizontal, ON bipolar, and Müller glial cells appear in small numbers after 90 days in culture (78). Retinal organoidogenesis with hiPSCs has been extremely limited. Parfitt et al. were successful at generating hiPSC-derived RECOVERIN+ optic cups by 13 weeks in culture based on the Nakano et al. differentiation and dissection protocol (77,88). Expression of mature photoreceptor markers appeared after 21 weeks in culture. A BRN3+/HuD+ ganglion layer was the only other mature neural retinal cell type observed, suggesting that the variance in timing and reproducibility with which retinal organoids generate all major retinal cell types remains a challenge.
Conclusions
The ability to differentiate iPSC into the various cell types of the retina has been useful for studying early retinal development and etiology, screening small compounds, testing gene replacement therapies, and providing source material for cell replacement therapies. Several protocols to generate various retinal cells have been developed to maximize a specific cell type or follow good manufacturing practice (GMP) guidelines for xeno-free therapeutic culture systems. Different retinal cells may be generated using varying protocols suitable for the intent of a particular study. While the efficiency at which these cells can be generated has increased significantly since the first spontaneous differentiations, several challenges still exist. For one, isolating pure cell populations from neural retinal differentiations remains difficult. Secondly, not all cell types or cell structures are generated with equal efficiency. Muller glia, the last cell type to differentiate during in vivo retinogenesis, appear in low yields after ~90–180 days in 3D-organiod culture. Additionally, only ~3% of cells that stain positive for mature photoreceptor markers develop outer segments after ~160 days in culture. As differentiation protocols continue to improve we are likely to see an increase in our basic understanding of various retinal degenerative diseases and the utilization of iPSC in clinical trials.
Acknowledgments
This work has been supported by Foundation for Fighting Blindness (FFB) and The Center for Advanced Retinal and Ocular Therapeutics (CAROT).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76. [Crossref] [PubMed]
- Kang L, Wang J, Zhang Y, et al. iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell 2009;5:135-8. [Crossref] [PubMed]
- Zhao XY, Li W, Lv Z, et al. iPS cells produce viable mice through tetraploid complementation. Nature 2009;461:86-90. [Crossref] [PubMed]
- Armstrong L, Tilgner K, Saretzki G, et al. Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells 2010;28:661-73. [Crossref] [PubMed]
- Hu BY, Weick JP, Yu J, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A 2010;107:4335-40. [Crossref] [PubMed]
- Choi J, Lee S, Mallard W, et al. A comparison of genetically matched cell lines reveals the equivalence of human iPSCs and ESCs. Nat Biotechnol 2015;33:1173-81. [Crossref] [PubMed]
- Data services and software for identifying genes and mutations causing retinal degeneration [database on the Internet]. Cadmus. 1998. Available online: http://www.sph.uth.tmc.edu/RetNet/. Accessed: February 3, 2017.
- Furukawa T, Kozak CA, Cepko CL. rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proc Natl Acad Sci U S A 1997;94:3088-93. [Crossref] [PubMed]
- Mathers PH, Grinberg A, Mahon KA, et al. The RX homeobox gene is essential for vertebrate eye development. Nature 1997;387:603-7. [Crossref] [PubMed]
- Voronina VA, Kozhemyakina EA, O'Kernick CM, et al. Mutations in the human RAX homeobox gene in a patient with anophthalmia and sclerocornea. Hum Mol Genet 2004;13:315-22. [Crossref] [PubMed]
- Rembold M, Loosli F, Adams RJ, et al. Individual cell migration serves as the driving force for optic vesicle evagination. Science 2006;313:1130-34. [Crossref] [PubMed]
- Zuber ME, Gestri G, Viczian AS, et al. Specification of the vertebrate eye by a network of eye field transcription factors. Development 2003;130:5155-67. [Crossref] [PubMed]
- Nguyen M, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development 2000;127:3581-91. [PubMed]
- Martínez-Morales JR, Dolez V, Rodrigo I, et al. OTX2 activates the molecular network underlying retina pigment epithelium differentiation. J Biol Chem 2003;278:21721-31. [Crossref] [PubMed]
- Rowan S, Chen CM, Young TL, et al. Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development 2004;131:5139-52. [Crossref] [PubMed]
- Horsford DJ, Nguyen MT, Sellar GC, et al. Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development 2005;132:177-87. [Crossref] [PubMed]
- Pittack C, Grunwald GB, Reh TA. Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development 1997;124:805-16. [PubMed]
- Hyer J, Mima T, Mikawa T. FGF1 patterns the optic vesicle by directing the placement of the neural retina domain. Development 1998;125:869-77. [PubMed]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev 2005;85:845-81. [Crossref] [PubMed]
- Quinn RH, Miller SS. Ion Transport Mechanisms in Native Human Retinal Pigment Epithelium. Investigative Ophthalmology and Visual Science 1992;33:3513-27. [PubMed]
- Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. Journal of Cell Biology 1969;42:392-403. [Crossref] [PubMed]
- Seagle B-LL, Rezai KA, Kobori Y, et al. Melanin photoprotection in the human retinal pigment epithelium and its correlation with light-induced cell apoptosis. Proc Natl Acad Sci U S A 2005;102:8978-83. [Crossref] [PubMed]
- Fulton BS, Rando RR. Biosynthesis of 11-cis-retinoids and retinyl esters by bovine pigment epithelium membranes. Biochemistry 1987;26:7938-45. [Crossref] [PubMed]
- Gehrs KM, Anderson DH, Johnson LV, et al. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med 2006;38:450-71. [Crossref] [PubMed]
- Kawasaki H, Suemori H, Mizuseki K, et al. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc Natl Acad Sci U S A 2002;99:1580-5. [Crossref] [PubMed]
- Haruta M, Sasai Y, Kawasaki H, et al. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest Ophthalmol Vis Sci 2004;45:1020-5. [Crossref] [PubMed]
- Klimanskaya I, Hipp J, Rezai KA, et al. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells 2004;6:217-45. [Crossref] [PubMed]
- Lund RD, Wang S, Klimanskaya I, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells 2006;8:189-99. [Crossref] [PubMed]
- Vugler A, Lawrence J, Walsh J, et al. Embryonic stem cells and retinal repair. Mech Dev 2007;124:807-29. [Crossref] [PubMed]
- Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigment epithelium from induced pluripotent stem cells. Stem Cells 2009;27:2427-34. [Crossref] [PubMed]
- Carr AJ, Vugler AA, Hikita ST, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One 2009;4:e8152. [Crossref] [PubMed]
- Ferguson LR, Balaiya S, Mynampati BK, et al. Deprivation of bFGF promotes spontaneous differentiation of human embryonic stem cells into retinal pigment epithelial cells. J Stem Cells 2015;10:159-70. [PubMed]
- Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell 2009;5:396-408. [Crossref] [PubMed]
- Jaffe GJ, Harrison CE, Lui GM, et al. Activin expression by cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 1994;35:2924-31. [PubMed]
- Fuhrmann S, Levine EM, Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development 2000;127:4599-609. [PubMed]
- Westenskow P, Sedillo Z, Barnett A, et al. Efficient derivation of retinal pigment epithelium cells from stem cells. J Vis Exp 2015. [Crossref] [PubMed]
- Zahabi A, Shahbazi E, Ahmadieh H, et al. A new efficient protocol for directed differentiation of retinal pigmented epithelial cells from normal and retinal disease induced pluripotent stem cells. Stem Cells Dev 2012;21:2262-72. [Crossref] [PubMed]
- Gerrard L, Rodgers L, Cui W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells 2005;23:1234-41. [Crossref] [PubMed]
- Chambers SM, Fasano CA, Papapetrou EP, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 2009;27:275-80. [Crossref] [PubMed]
- Meyer JS, Howden SE, Wallace KA, et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells 2011;29:1206-18. [Crossref] [PubMed]
- Okada Y, Shimazaki T, Sobue G, et al. Retinoic-acid-concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev Biol 2004;275:124-42. [Crossref] [PubMed]
- Perron M, Boy S, Amato MA, et al. A novel function for Hedgehog signalling in retinal pigment epithelium differentiation. Development 2003;130:1565-77. [Crossref] [PubMed]
- Macdonald R, Barth KA, Xu Q, et al. Midline signaling is required for Pax gene regulation and patterning of the eyes. Development 1995;121:3267-78. [PubMed]
- Spence JR, Madhavan M, Ewing JD, et al. The hedgehog pathway is a modulator of retina regeneration. Development 2004;131:4607-21. [Crossref] [PubMed]
- Buchholz DE, Pennington BO, Croze RH, et al. Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl Med 2013;2:384-93. [Crossref] [PubMed]
- Leach LL, Buchholz DE, Nadar VP, et al. Canonical/beta-catenin Wnt pathway activation improves retinal pigmented epithelium derivation from human embryonic stem cells. Invest Ophthalmol Vis Sci 2015;56:1002-13. [Crossref] [PubMed]
- Leach LL, Croze RH, Hu Q, et al. Induced Pluripotent Stem Cell-Derived Retinal Pigmented Epithelium: A Comparative Study Between Cell Lines and Differentiation Methods. J Ocul Pharmacol Ther 2016;32:317-30. [Crossref] [PubMed]
- Maruotti J, Sripathi SR, Bharti K, et al. Small-molecule–directed, efficient generation of retinal pigment epithelium from human pluripotent stem cells. Proc Natl Acad Sci U S A 2015;112:10950-5. [Crossref] [PubMed]
- Kung AL, Zabludoff SD, France DS, et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell 2004;6:33-43. [Crossref] [PubMed]
- Lane A, Philip LR, Ruban L, et al. Engineering efficient retinal pigment epithelium differentiation from human pluripotent stem cells. Stem Cells Transl Med 2014;3:1295-304. [Crossref] [PubMed]
- Cyranoski D. Stem cells cruise to clinic: Japanese study of induced pluripotent stem cells aims to demonstrate safety in humans. Nature 2013;494:413. [Crossref] [PubMed]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci 2001;2:109-18. [Crossref] [PubMed]
- Harada T, Harada C, Parada LF. Molecular regulation of visual system development: more than meets the eye. Genes Dev 2007;21:367-78. [Crossref] [PubMed]
- Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer 2011;12:23-38. [PubMed]
- Baye LM, Link BA. Interkinetic nuclear migration and the selection of neurogenic cell divisions during vertebrate retinogenesis. J Neurosci 2007;27:10143-52. [Crossref] [PubMed]
- Enver T, Soneji S, Joshi C, et al. Cellular differentiation hierarchies in normal and culture-adapted human embryonic stem cells. Hum Mol Genet 2005;14:3129-40. [Crossref] [PubMed]
- Cereso N, Pequignot MO, Robert L, et al. Proof of concept for AAV2/5-mediated gene therapy in iPSC-derived retinal pigment epithelium of a choroideremia patient. Mol Ther Methods Clin Dev 2014;1:14011. [Crossref] [PubMed]
- Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet 1997;13:157-62. [Crossref] [PubMed]
- Piccolo S, Sasai Y, Lu B, et al. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell 1996;86:589-98. [Crossref] [PubMed]
- Glinka A, Wu W, Onichtchouk D, et al. Head induction by simultaneous repression of Bmp and Wnt signaling in Xenopus. 1997;389:517-9.
- Lee H-Y, Klèber M, Hari L, et al. Instructive role of Wnt/β-catenin in sensory fate specification in neural crest stem cells. Science 2004;303:1020-3. [Crossref] [PubMed]
- Liu W, Lagutin O, Swindell E, et al. Neuroretina specification in mouse embryos requires Six3-mediated suppression of Wnt8b in the anterior neural plate. J Clin Invest 2010;120:3568-77. [Crossref] [PubMed]
- Lamba DA, Karl MO, Ware CB, et al. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A 2006;103:12769-74. [Crossref] [PubMed]
- Lamba DA, McUsic A, Hirata RK, et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One 2010;5:e8763. [Crossref] [PubMed]
- Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol 2008;26:215-24. [Crossref] [PubMed]
- Osakada F, Jin ZB, Hirami Y, et al. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci 2009;122:3169-79. [Crossref] [PubMed]
- Mellough CB, Sernagor E, Moreno-Gimeno I, et al. Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem Cells 2012;30:673-86. [Crossref] [PubMed]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A 1979;76:514-7. [Crossref] [PubMed]
- Brewer GJ, Torricelli JR, Evege EK, et al. Optimized survival of hippocampal neurons in B27-supplemented neurobasalTM, a new serum-free medium combination. J Neurosci Res 1993;35:567-76. [Crossref] [PubMed]
- Svendsen CN, Fawcett JW, Bentlage C, et al. Increased survival of rat EGF-generated CNS precursor cells in B27 supplemented medium. Exp Brain Res 1995;102:407-14. [Crossref] [PubMed]
- Chen Y, Stevens B, Chang J, et al. NS21: re-defined and modified supplement B27 for neuronal cultures. J Neurosci Methods 2008;171:239-47. [Crossref] [PubMed]
- Piccolo S, Agius E, Leyns L, et al. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP, and Wnt signals. Nature 1999;397:707-10. [Crossref] [PubMed]
- Smith JC, Price BMJ, Van Nimmen K, et al. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature 1990;345:729-31. [Crossref] [PubMed]
- Bell E, Muñoz-Sanjuán I, Altmann CR, et al. Cell fate specification and competence by Coco, a maternal BMP, TGFbeta and Wnt inhibitor. Development 2003;130:1381-9. [Crossref] [PubMed]
- Zhou S, Flamier A, Abdouh M, et al. Differentiation of human embryonic stem cells into cone photoreceptors through simultaneous inhibition of BMP, TGFbeta and Wnt signaling. Development 2015;142:3294-306. [Crossref] [PubMed]
- Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011;472:51-6. [Crossref] [PubMed]
- Nakano T, Ando S, Takata N, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 2012;10:771-85. [Crossref] [PubMed]
- Kaewkhaw R, Kaya KD, Brooks M, et al. Transcriptome Dynamics of Developing Photoreceptors in Three-Dimensional Retina Cultures Recapitulates Temporal Sequence of Human Cone and Rod Differentiation Revealing Cell Surface Markers and Gene Networks. Stem Cells 2015;33:3504-18. [Crossref] [PubMed]
- Völkner M, Zschätzsch M, Rostovskaya M, et al. Retinal Organoids from Pluripotent Stem Cells Efficiently Recapitulate Retinogenesis. Stem Cell Reports 2016;6:525-38. [Crossref] [PubMed]
- Hyer J, Kuhlman J, Afif E, et al. Optic cup morphogenesis requires pre-lens ectoderm but not lens differentiation. Developmental Biology 2003;259:351-63. [Crossref] [PubMed]
- Gonzalez-Cordero A, West EL, Pearson RA, et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol 2013;31:741-7. [Crossref] [PubMed]
- Decembrini S, Koch U, Radtke F, et al. Derivation of traceable and transplantable photoreceptors from mouse embryonic stem cells. Stem Cell Reports 2014;2:853-65. [Crossref] [PubMed]
- Bae D, Mondragon-Teran P, Hernandez D, et al. Hypoxia enhances the generation of retinal progenitor cells from human induced pluripotent and embryonic stem cells. Stem Cells Dev 2012;21:1344-55. [Crossref] [PubMed]
- Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 2014;9:2329-40. [Crossref] [PubMed]
- Reichman S, Terray A, Slembrouck A, et al. From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc Natl Acad Sci U S A 2014;111:8518-23. [Crossref] [PubMed]
- Jadhav AP, Mason HA, Cepko CL. Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development 2006;133:913-23. [Crossref] [PubMed]
- Yaron O, Farhy C, Marquardt T, et al. Notch1 functions to suppress cone-photoreceptor fate specification in the developing mouse retina. Development 2006;133:1367-78. [Crossref] [PubMed]
- Parfitt DA, Lane A, Ramsden CM, et al. Identification and Correction of Mechanisms Underlying Inherited Blindness in Human iPSC-Derived Optic Cups. Cell Stem Cell 2016;18:769-81. [Crossref] [PubMed]
Cite this article as: Weed LS, Mills JA. Strategies for retinal cell generation from human pluripotent stem cells. Stem Cell Investig 2017;4:65.


