Histone chaperone in regulation of cellular metabolism dictating stem cell fate?
Cellular metabolism could regulate pluripotent states of embryonic stem cells (ESC) mediated by metabolites (1). These metabolites simultaneously could dictate the fate of pluripotent states through epigenetic modifications. Dynamicity in expression of three different metabolites namely, acetate, S-adenosylmethionine and O-linked β-N-acetylglucosamine facilitate the regulation of epigenetic landscape and thus influence the fate of ESCs and the pluripotent states (1). Human ESCs and mouse EpiESCs exist in a primed state of pluripotency in comparison to the naïve state of pluripotency in mouse ESCs (2). Colony morphologies, response to signaling pathways, contribution to generate chimera, epigenetic states and cellular metabolism distinctively divides these two states of pluripotency (2). Primed ESCs predominantly utilize glycolysis whereas naïve ESCs exploit a bivalent metabolism, switching between glycolysis and oxidative phosphorylation on demand as major source of energy (3). α-Ketoglutarate (α-KG), an intermediate product of TCA cycle, has been shown to enhance pluripotency in mouse ESCs while inhibit differentiation by blocking DNA and histone demethylation (4). But, in hESCs, increased α-KG level accelerated initial differentiation while an induced succinate level delayed differentiation, implying a context specific role in cell fate determination (5). Metabolites, including α-KG, serve as cofactor to regulate dioxygenase enzymes associated with epigenetic modifications. Exploiting the concept of metabolism, small molecules targeting metabolic pathways have been proved to rescue mitochondrial DNA mutation by manipulation of pluripotent stem cells (6). So, a better understanding in regulation of pluripotency by epigenetic modification through metabolites will further enrich the field of regenerative medicine.
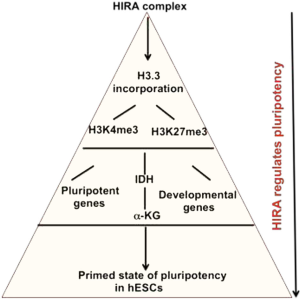
Till now, the role of metabolites were limited as upstream regulators to epigenetic modifications, but this new study by Zhu et al. demonstrated the existence of a master regulator above this axis guiding the metabolite mediated epigenetic regulation of human ESCs pluripotency (7). Histone cell cycle regulation-defective homolog A (HIRA) is a histone chaperone and this study has assigned it as a player controlling the metabolite related epigenetic regulation of hESCs state (7). HIRA, resembling other histone chaperones, aid in histone metabolism, DNA repair, and replication and has been associated with transcriptional regulation as well (8). In a replication-uncoupled manner, HIRA complex comprising of HIRA, UBN1 and CABIN1 deposit histone variant H3.3 at chromatin regions resulting in activation by destabilization of the nucleosome at the TSSs (9). But, Zhu et al. reported the involvement of HIRA in cellular metabolism for the first time, which further established a classic example on the difference in the pluripotent states of mouse and human ESCs (7). This study has substantially contributed towards the understanding of epigenetic-metabolic regulatory axis in stem cells.
Based on a high-throughput siRNA screen with the human siGENOME siRNA Library-Transcription factors-SMART pool using siRNAs against OCT4 and EGFP, Zhu et al. screened 25 candidate genes wherein they selected Prohibitin (PHB) protein, due to a high z-score in the siRNA study, significant changes in the morphology of ESCs upon its knockdown and most importantly, its function remained elusive in human ESCs till date (7). Proteomics analysis in undifferentiated mESCs showed PHB2 as one of the highly expressed candidate gene (10) and ablation of PHB2 led to huge apoptosis and early embryonic lethality in mice (11,12). But, PHB could not influence the proliferative capacity of hESCs in comparison to mESCs (7). This difference could be attributed to the localization of PHB in mouse vs. human ESCs due to the altered pluripotent states.
In this study, transcriptome analysis of PHB-knocked down hESCs revealed changes associated with chromatin modifiers, glucose metabolic processes, mRNA processing along with the influence on lineage-specific and pluripotency related genes. Increased level of histone mono-, di-, tri-methylated H3K4 and H3K27 along with H3K36me3 upon PHB downregulation indicated the role of PHB in retaining the chromatin landscape prevalent in hESCs. Even PHB-knockdown in human fibroblasts significantly reduced the reprogramming potential of these cells. So, PHB could effectively maintain and retain the pluripotent state of hESCs (7). Role of PHB in cellular reprogramming could be exploited further to improve the reprogramming of human fibroblasts.
Although nuclear and cytosolic expression has been well evidenced, PHB is predominantly localized to mitochondria. So, how PHB could regulate the fate of hESCs by regulating epigenetic modifications associated with chromatin in the nucleus? Which is the driving force or downstream effector of PHB? Zhu et al. showed that along with other mitochondrial proteins, PHB interacted with all components of the HIRA complex including HIRA, CABIN1 and UBN1. Functionally, knockdown of PHB reduced their expression at the protein level but not at the transcript level. So, basically, Zhu et al. inferred that PHB stabilizes the HIRA protein complex in hESCs and thereby regulate its function. Time course analysis showed that stabilization of the HIRA complex is the earliest event for the regulation of hESC self-renewal by the HIRA-PHB axis. Expectedly, HIRA knockdown in hESCs demonstrated similar changes as observed in PHB-deficient hESCs (7).
Downregulation of HIRA in hESCs resulted in a similar histone methylation pattern as in absence of Isocitrate dehydrogenase (IDH), the enzyme responsible for conversion of Isocitrate to α-KG, in hESCs (13). Zhu et al. showed that knockdown of HIRA, reduced the expression of IDH genes (different isoforms) resulting in reduced production of α-KG that could dictate the maintenance of hESC pluripotent state as a downstream effector of HIRA. In fact, upon supplemented with cell permeable α-KG, HIRA-kd hESCs could rescue the defect. Interestingly, a similar effect of α-KG was observed in mouse ESCs (4). But, earlier studies demonstrated that α-KG could induce initial differentiation in both primed hESCs and mouse EpiSCs (5).
Zhu et al. demonstrated that the genome-wide H3.3 incorporation in hESCs was significantly decreased in absence of HIRA, which is also extensively supported by previous studies (9), although the extent of enrichment varied within different sites of the chromatin. Bivalent promoter is a mark of pluripotent ESCs (14). In this study also, deprivation of HIRA resulted in enhanced incorporation of active H3K4me3 mark at the developmental genes while depleted level at the pluripotency and IDH genes (7). Incorporation of the repressive H3K27me3 mark was enhanced at the pluripotency genes but depleted within the lineage specific genes (7). No significant loss or enrichment in H3K27me3 level was observed at the IDH genes.
Thus, Zhu et al. established a fundamental aspect of stem cell biology for the first time in studying the regulation of metabolism by histone chaperone. It also pointed out again a major difference in the pluripotent state of mouse vs. human ESCs. HIRA is dispensable for pluripotency in mESCs whereas this study proved that HIRA is required to maintain the self-renewal status in hESCs. Also, it would be interesting to see how PHB contribute towards retention of naïve state of mouse ESC. Earlier report indicated that PHB2 is associated with the proliferation of mESCs and its downregulation induced differentiation of mESCs (9). But, further studies are needed to unravel the regulation of HIRA by PHB and their subsequent effect on the naïve state of pluripotency. This study established that α-KG associate with pluripotent nature of both naïve and primed pluripotency, which is rather a deviation in the context of distinct differences in characteristics within two pluripotent states. Although, hESCs cultured in naïve condition would have presented a clearer picture.
Use of hESCs in regenerative medicine demands perfect differentiation of the cells to be used for patients. So, a better understanding on the regulation of stem cell fate could further potentiate the differentiation strategies ensuring the generation of stable progenitor cells. It could be inferred from this study that, HIRA, positioning itself at the top of the pyramid, could be targeted as small molecule affecting metabolite mediated epigenetic regulation of pluripotency of hESCs (Figure 1).
Acknowledgements
D Dutta is financially supported by the institute, aided by Department of Biotechnology, India.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Moussaieff A, Kogan NM, Aberdam D. Concise Review: Energy Metabolites: Key Mediators of the Epigenetic State of Pluripotency. Stem Cells 2015;33:2374-80. [Crossref] [PubMed]
- Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell 2009;4:487-92. [Crossref] [PubMed]
- Sperber H, Mathieu J, Wang Y, et al. The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nat Cell Biol 2015;17:1523-35. [Crossref] [PubMed]
- Carey BW, Finley LW, Cross JR, et al. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 2015;518:413-6. [Crossref] [PubMed]
- TeSlaa T, Chaikovsky AC, Lipchina I, et al. α-Ketoglutarate Accelerates the Initial Differentiation of Primed Human Pluripotent Stem Cells. Cell Metab 2016;24:485-93. [Crossref] [PubMed]
- Ma H, Folmes CD, Wu J, et al. Metabolic rescue in pluripotent cells from patients with mtDNA disease. Nature 2015;524:234-8. [Crossref] [PubMed]
- Zhu Z, Li C, Zeng Y, et al. PHB Associates with the HIRA Complex to Control an Epigenetic-Metabolic Circuit in Human ESCs. Cell Stem Cell 2017;20:274-289.e7. [Crossref] [PubMed]
- Hammond CM, Strømme CB, Huang H, et al. Histone chaperone networks shaping chromatin function. Nat Rev Mol Cell Biol 2017;18:141-58. [Crossref] [PubMed]
- Pchelintsev NA, McBryan T, Rai TS, et al. Placing the HIRA histone chaperone complex in the chromatin landscape. Cell Rep 2013;3:1012-9. [Crossref] [PubMed]
- Kowno M, Watanabe-Susaki K, Ishimine H, et al. Prohibitin 2 regulates the proliferation and lineage-specific differentiation of mouse embryonic stem cells in mitochondria. PLoS One 2014;9:e81552. [Crossref] [PubMed]
- Merkwirth C, Dargazanli S, Tatsuta T, et al. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev 2008;22:476-88. [Crossref] [PubMed]
- Merkwirth C, Langer T. Prohibitin function within mitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim Biophys Acta 2009;1793:27-32.
- Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 2011;19:17-30. [Crossref] [PubMed]
- Voigt P, Tee WW, Reinberg D. A double take on bivalent promoters. Genes Dev 2013;27:1318-38. [Crossref] [PubMed]
Cite this article as: Dutta D. Histone chaperone in regulation of cellular metabolism dictating stem cell fate? Stem Cell Investig 2017;4:50.


