The propensity for epithelial-mesenchymal transitions is dictated by chromatin states in the cancer cell of origin
Epithelial-mesenchymal transition (EMT) drives tumor progression and metastasis
At its core, epithelial-mesenchymal transition (EMT) programs are well-established physiological processes that transpire during embryogenesis to facilitate the development of the mesoderm and neural crest, and during adulthood to oversee the repair of wounded tissues (1-3). Epithelial cells undergoing EMT programs downregulate epithelial markers (e.g., E-cadherin) and upregulate mesenchymal markers (e.g., vimentin), leading to stark changes in cell morphology and behavior. For instance, epithelial cells are typically arranged as a single layer of polarized cells that exhibit strong cell-cell contacts, a phenotype that gives way during EMT programs to the generation of apolar mesenchymal-like cells that exhibit elongated spindle morphologies (1-3). Importantly, the phenomenon of EMT can be hijacked by pathological processes, including fibrosis, inflammatory conditions, and the progression and metastasis of solid tumors (1). During pathological EMT programs, carcinoma cells within a growing primary tumor shed their epithelial phenotypes and transition towards a more mobile and invasive mesenchymal phenotype that enables disease progression and metastasis (2,4-6). Indeed, the appearance of sarcomatoid morphologies in post-EMT carcinoma cells portends to disease progression and the dissemination of metastatic cells to distant locales (2,4,5). Furthermore, EMT programs also promote cancer cell survival through the combined actions of both cell intrinsic and extrinsic mechanisms that culminate in drug resistance and the generation of carcinoma cells bearing cancer stem cell-like properties.
The process of EMT is mediated by a number of cytokines and growth factors, the most notable of which is transforming growth factor-β (TGF-β) (1,7). TGF-β binds to the TGF-β Type II Receptor (TβR-II that in turn recruits, transphosphorylates, and stimulates the TGF-β Type I Receptor TβR-I). Once activated, this ternary receptor complex binds and activates Smad2/3, which complexes with Smad4, leading to their nuclear translocation and association with an array of transcription factors that govern the expression of target genes (1,8). Altered expression of these TGF-β-responsive genes serve to induce phenotypic changes associated with EMT programs and their initiation of tumor progression and metastasis. EMT programs also manifest the “TGF-β Paradox,” which refers to the ability of tumorigenesis and EMT programs to convert the functions of TGF-β from that of a tumor suppressor to a tumor promoter (1,7); they also induce tumor cells to acquire stem cell-like properties, including acquisition of stem cell markers, increased sphere forming capacity, and tumor initiation potential (9,10). Indeed, while numerous tumor cells readily escape the primary tumor on a daily basis (e.g., 1 million cells/day), only those capable of tumor initiation, cancer stem cells (CSCs), ultimately produce overt metastases (11-13). CSCs maintain unlimited self-renewal capacity and the ability to differentiate into tumor cells that comprise the bulk of primary tumors and their satellite metastatic tumor nodules. Importantly, CSCs also possess properties associated with de novo and acquired resistance to chemotherapeutics, a trait that imparts significant challenges associated with targeting and killing CSCs (8). Recent studies suggest that acquired chemotherapeutic resistance of CSCs is a result of epigenetic changes that readily accumulate in these cells when confronted with standard-of-care anticancer agents (14,15).
Epigenetic modulation of EMT: Chromatin states prime CSCs for EMT
As mentioned above, carcinoma cells traversing the EMT program lose their epithelial cell polarity and cell-cell adhesions and acquire mesenchymal traits, such as the ability to invade and metastasize (1). While the transcriptomic and phenotypic changes associated with these events have been extensively documented, an emerging area of EMT research focuses on the extrinsic signals that drive the reprogramming of the epigenome. Recent evidence suggests that the tumor microenvironment (TME) signals EMT induction by activating transcription factors associated with EMT [e.g., SNAIL, SLUG, ZEB1, and TWIST (9)]. Loss of cell-cell adhesions through the inactivation of E-cadherin, a critical adherens junction protein, is a hallmark of the EMT process (9,16). Moreover, master EMT transcription factors induced by TME perturbations function to inactivate E-cadherin primary through epigenetic silencing (9). At present, the means by which epigenomic alterations impact cell fate decisions, including susceptibility to undergo EMT programs, remain to be fully elucidated.
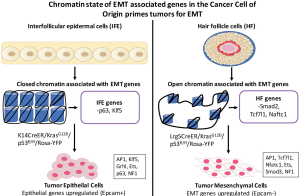
In an attempt address the aforementioned question, Latil and colleagues (17) determined in their recent Cell Stem Cell paper how unique chromatin states in the cancer cell of origin dictate the heterogeneity and behavior of developing squamous cell carcinomas (SCC), as well as their capacity to undergo EMT programs. Previous studies showed that the expression of oncogenic KRasG12D coupled with genetic deletion of p53 deletion in interfollicular epidermal (IEF) cells was sufficient to elicit SCC with well-differentiated morphology; however, recapitulating these same genetic events in hair follicle (HF) cells resulted in SCC bearing multiple morphologies, including well-differentiated SCC, spindle cell SCC, and mixed morphology SCC (18,19). Such findings suggest that cell of origin and its unique genetic makeup ultimately determines the tumorigenic fate of distinct cell types to identical genetic events. To test this supposition specifically within IEF and HF epidermal cells, the authors utilized two genetic targeting strategies: (I) the K14-promoter to express CreER preferentially within IFE cells to yield K14CreER/KRasG12D/p53fl/fl/Rosa-YFP transgenic mice; and (II) the Lrg5-promoter to express CreER preferentially within HF stem cells to yield Lrg5CreER/KRasG12D/p53fl/fl/Rosa-YFP transgenic mice. In doing so, Latil and colleagues noticed that both K14CreER and Lrg5CreER mice formed tumors that exhibited similar growth and latency rates, typically appearing at ~6-9 weeks of age. Interestingly, K14CreER mice developed almost exclusively well-differentiated SCC that expressed robust levels of epithelial markers, while Lrg5CreER mice developed more numerous tumors with three distinct cellular morphologies: (I) well-differentiated SCC that resembled those of their K14CreER counterparts and contained keratin pearls; (II) mesenchymal SCCs that expressed robust levels of mesenchymal markers; and (III) mixed SCC tumors that represented the most frequently observed SCC tumor and comprised a mixture of well-differentiated and mesenchymal tumor cells. Given the propensity of post-EMT cells to metastasize, Latil and colleagues hypothesized that HF-derived Tumor Mesenchymal Cells (TMCs) would be more likely to form metastases than their IFE-derived Tumor Epithelial Cells (TECs) counterparts. Accordingly, intravenous injection of TMC and TEC derivatives into mice borne out this prediction, suggesting that cancer cell of origin primes tumor cells to undergo EMT and, consequently, dictates their propensity to metastasize.
In an effort to identify the mechanisms whereby EMT priming transpires in the cancer cell of origin, Latil et al. profiled the transcriptomes of both IFE and HF cells, as well as tumors derived from their transformation in the K14CreER and Lrg5CreER mice. Gene set enrichment analysis (GSEA) showed enhanced expression of genes operant in regulating epithelial states in both normal IFE cells, as well as in their TEC Epcam+ counterparts. Interestingly, 29% of the genes determined to be upregulated in TEC cells were already expressed robustly in normal IFE cells as compared to HF cells. Amongst the genes coincidently expressed in IFE and TEC cells were the epithelial transcription factors, such as p63, Ovol, Grhl, Cebpa, and Klf5. In stark contrast, GSEA of normal HF and TMC Epcam− cells demonstrated that these cell types were highly enhanced in the expression of EMT-associated genes. As above, 27% of the genes found to be upregulated in TMC cells were highly expressed in normal HF cells. Included amongst the EMT-associated genes coincidently expressed between HF and TMC cells were Ltbp2, Grem1, Flstl1, S100A4, Nfatc1, Tbx1, Tcf4, Tcf7l1, and Ctgf. Collectively, these analyses served to strengthen the notion that basal transcriptional profiles housed within the cancer cell of origin influences the tumorigenicity of their malignant prodigy, as well as their ability to undergo EMT programs.
To extend the aforementioned conclusions, the authors undertook the performance of Assay for Tansposase-Accessible Chromatin sequencing. In doing so, Latil et al. identified how chromatin landscapes change during both tumorigenesis, as well as during EMT programs. As expected, open chromatin regions were identified in genes commonly associated with tumor initiation. Consistent with their transcriptomic profiles analyses described above, Latil and colleagues determined that 139 EMT-responsive genes localized to regions of open chromatin in HF cells, suggesting that epigenetic priming determines cellular propensity to undergo EMT programs. Indeed, TGF-β stimulation of EMT in TMCs readily induced their expression of Jun/AP1, NF1, Ets1, bHLH TGs, Nfatc, and Smad2. Moreover, the authors found that the expression of p63, a gene typically involved in regulating epithelial cell homeostasis, was responsible for suppressing epigenetic and transcriptomic reprogramming coupled to EMT programs in IFE-derived tumors. Along these lines, p63 normally antagonizes oncogenic TGF-β signaling and its stimulation of tumor development and metastatic progression. However, mutant p53 can form a ternary complex with TGF-β-regulated Smads and p63, thereby inactivating the tumor suppressing activities of p63 and enabling the tumor promoting properties of TGF-β, including its stimulation of metastasis and CSC self-renewal (20,21).
Taken together, the findings (Figure 1) presented by Latil and colleagues demonstrate how chromatin states within cancer cell of origin dictate carcinoma cell propensity to undergo EMT programs; they also highlight the importance of deciphering the role of epigenetic modifications in driving disease progression and metastasis.
Conclusions and future directions
Mesenchymal-epithelial transitions (MET) represent a reversion to epithelial states following the EMT process; it is also an essential component in the outgrowth and development of secondary metastatic lesions. Indeed, in the absence of continual TME-derived signals, carcinoma cells will readily assume epithelial phenotypes via MET (9). Thus, it stands to reason that MET programs are equally essential in facilitating metastatic outgrowth by disseminated post-EMT carcinoma cells. It is interesting to note that HF-derived TMC metastases appear to be locked into a perpetual EMT state, a condition that has been associated with reduced metastatic competency of SCCs (1,7,22). Thus, future studies need to investigate whether the multiple tumor morphologies noted in HF-derived tumors represent distinct tumor subtypes versus distinct stages of disease progression, as well as the extent to which these events are malleable epigenetically and subject to MET programs.
The gene regulatory network (GRN) developed by Latil and colleagues is hypothesized to predict for EMT programs. As such, Latil et al. investigated the importance of the TGF-β/Smad2 signaling axis in stimulating EMT programs in developing and progressing SCCs. It is interesting to note that the “TGF-β Paradox” was first demonstrated genetically in a mouse model of SCC. Indeed, Cui et al. (23) demonstrated that transgenic expression of TGF-β1 within the epidermis of mice dramatically inhibited carcinogen-induced tumor formation; however, once formed, TGF-β1 rapidly drove the progression and aggressiveness of these spindle cell SCCs, a reaction that coincided with the activation of EMT programs. In the current study, Latil and colleagues focused on the importance of Smad2 in driving SCC progression in response to TGF-β. Teleologically, this is an interesting finding for two reasons. First, EMT programs stimulated by TGF-β commonly reflect its activation of Smad3, not Smad2, and second, Smad2 is incapable of binding to DNA. Thus, future studies need to determine the relative involvement of Smads 3 and 4 in mediating HF/TMC-derived tumor development, as well as identify the Smad2-interacting proteins responsible for localizing this effector molecule to open chromatin states. Likewise, given the importance of noncanonical TGF-β signaling systems in promoting its oncogenic activity (24), it therefore seems appropriate to assess the relative contribution of noncanonical TGF-β effectors to epigenetic priming of cancer cell of origin, including their capacity to inactivate the gatekeeper function of p63 (20).
Finally, CSCs underlie tumor heterogeneity, but the cancer cell of origin appears to give rise to distinct populations of tumors dependent upon their chromatin states and epigenetic priming. This raises the question of how EMT priming by the cancer cell of origin relates to tumor heterogeneity in EMT-dependent tumors, particularly their evolution and progression. As such, future studies should address the importance of epigenetic priming in CSCs, as well as how these chromatin states contribute to the tumorigenicity of CSCs. Ultimately, answering these intriguing questions will build upon this impressive study and will provide the necessary foundation to employ precision medicine approaches against the cancer cell of origin and its unique chromatin state.
Acknowledgements
Members of the Schiemann Laboratory are thanked for critical reading of the manuscript. Support was provided in part by the National Institutes of Health (CA129359, CA177069, and CA194518) and METAvivor to W.P.S., and by the Molecular Therapeutics Training Program (5T32GM008803-12) to A.A.L.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wendt MK, Allington TM, Schiemann WP. Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol 2009;5:1145-68. [Crossref] [PubMed]
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 2008;14:818-29. [Crossref] [PubMed]
- Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol 2008;19:294-308. [Crossref] [PubMed]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119:1420-8. [Crossref] [PubMed]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15:178-96. [Crossref] [PubMed]
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell 2006;127:679-95. [Crossref] [PubMed]
- Morrison CD, Parvani JG, Schiemann WP. The relevance of the TGF-beta Paradox to EMT-MET programs. Cancer Lett 2013;341:30-40. [Crossref] [PubMed]
- Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010;29:4741-51. [Crossref] [PubMed]
- Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med 2013;19:1438-49. [Crossref] [PubMed]
- Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704-15. [Crossref] [PubMed]
- Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell 2012;10:717-28. [Crossref] [PubMed]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008;8:755-68. [Crossref] [PubMed]
- Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med 2011;17:313-9. [Crossref] [PubMed]
- Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta 2009;1796:75-90.
- Sharma SV, Lee DY, Li B, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 2010;141:69-80. [Crossref] [PubMed]
- Graff JR, Greenberg VE, Herman JG, et al. Distinct patterns of E-cadherin CpG island methylation in papillary, follicular, Hurthle's cell, and poorly differentiated human thyroid carcinoma. Cancer Res 1998;58:2063-6. [PubMed]
- Latil M, Nassar D, Beck B, et al. Cell-Type-Specific Chromatin States Differentially Prime Squamous Cell Carcinoma Tumor-Initiating Cells for Epithelial to Mesenchymal Transition. Cell Stem Cell 2017;20:191-204.e5.
- Lapouge G, Youssef KK, Vokaer B, et al. Identifying the cellular origin of squamous skin tumors. Proc Natl Acad Sci U S A 2011;108:7431-6. [Crossref] [PubMed]
- White AC, Tran K, Khuu J, et al. Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proc Natl Acad Sci U S A 2011;108:7425-30. [Crossref] [PubMed]
- Adorno M, Cordenonsi M, Montagner M, et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell 2009;137:87-98. [Crossref] [PubMed]
- Chakrabarti R, Wei Y, Hwang J, et al. DeltaNp63 promotes stem cell activity in mammary gland development and basal-like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat Cell Biol 2014;16:1004-15, 1-13.
- Tsai JH, Donaher JL, Murphy DA, et al. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 2012;22:725-36. [Crossref] [PubMed]
- Cui W, Fowlis DJ, Bryson S, et al. TGFbeta1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell 1996;86:531-42. [Crossref] [PubMed]
- Parvani JG, Taylor MA, Schiemann WP. Noncanonical TGF-beta signaling during mammary tumorigenesis. J Mammary Gland Biol Neoplasia 2011;16:127-46. [Crossref] [PubMed]
Cite this article as: La Belle AA, Schiemann WP. The propensity for epithelial-mesenchymal transitions is dictated by chromatin states in the cancer cell of origin. Stem Cell Investig 2017;4:44.


