Immune checkpoint blockade for hematologic malignancies: a review
Introduction
Immune evasion is a hallmark of cancer (1). This process can be reversed via drugs that block immune checkpoints and bolster endogenous antitumor immune responses as evidenced by the success of CTLA-4 and PD-1 pathway blocking antibodies in melanoma, lung cancer, renal cell carcinoma, and other solid tumors (2). In this review, we discuss the current state of immunotherapeutic drug development in hematologic malignancies (HM) focused on targeting of immune checkpoints and the tumor microenvironment (TME), potential mechanisms of resistance to checkpoint blockade, and possible strategies for expanding the number of patients with HM who benefit from immune checkpoint directed therapies.
Biology of the immune checkpoints: focus on PD-1 and CTLA-4
Humans have evolved to maintain immune homeostasis through the use of multiple overlapping mechanisms aimed at preventing autoimmunity. In the adaptive immune system this requires a balance between recognition of non-self-antigen epitopes by naive T cell clones and the avoidance of recognition of self, a process that begins through positive and negative selection of developing T-cells in the thymus. Naïve T-cell activation initiates in the lymphatic system and hinges on T-cell receptor (TCR) recognition of antigen in the context of major histocompatibility molecules (MHC) and effective co-stimulation of CD28 by CD80/86 on antigen presenting cells (APCs). This in turn results in CTLA-4 upregulation on the T cell, and through competitive binding to the co-stimulatory molecules CD80/CD86, negatively modulates activated T cells (3).
In the tissues, the interaction of the programmed cell death 1 receptor (PD-1, CD279) on activated T-cells with its ligands PD-L1 (B7-H1 or CD274) and PD-L2 (B7-DC or CD273) maintains immunologic tolerance through the suppression of auto-reactive T-cells. The clinical activity observed with PD-1 pathway blockade highlights its importance in tumor immune evasion and has led to clinical development of numerous antibodies that block the PD-1/PD-L1 pathway. APCs and tumor cells expressing PD-L1 can engage PD-1 on T cells resulting in T cell dysfunction and protection of PD-L1-expressing cells from T-cell mediated lysis (4,5) and in HMs tumor cell expression of PD ligands may be an inherent feature of disease biology (6). Although, identification of tumor types with PD-L1 expression in the TME identifies subsets of patients who benefit from checkpoint blockade, PD-1 ligand expression does not guarantee a response nor does its absence exclude the possibility of response to checkpoint blockade (7).
Clinical trials of immune checkpoint blockade in HM
CTLA-4 blockade
Ipilimumab and tremelimumab are two anti-CTLA-4 humanized IgG blocking antibodies currently in various stages of clinical development in solid tumor and HM (8,9). Ipilimumab led to clinical responses in metastatic melanoma leading to its FDA approval (10) and showed proof of concept for immune checkpoint blockade as a relevant strategy for drug development in oncology, which has subsequently been explored in solid tumors and multiple HM.
PD-1 blockade
PD-1 pathway blockade with nivolumab (11-15), pembrolizumab (16,17), atezolizumab (18), and durvalumab (MEDI4736) (19,20) has demonstrated activity in multiple solid tumor malignancies. Nivolumab and pembrolizumab are the two anti-PD1 agents in the most advanced stages of clinical development in HM. There are multiple additional agents designed to block PD-1 or PD-L1, which are in various earlier phases of clinical development.
PD-1 blockade in classical Hodgkin lymphoma (cHL)
TME in HL is composed of a dense-but-ineffective inflammatory infiltrate that is recruited to the tumor site by small numbers of Hodgkin Reed-Sternberg (HRS) cells (21). Near-universal genetic changes in chromosome locus 9p24.1 with corresponding PD-1 ligand upregulation through JAK-STAT signaling not only suggested a rationale for testing anti-PD-1 therapy, but appear to be a biologic determinant of presentation and survival in cHL (6). In a series of 108 biopsy specimens from patients with newly diagnosed cHL, 105 (97%) had increased expression of PD-1 ligands detected using immunohistochemistry (IHC) (6). In another 246 patient series with HL, PD-L1 expression by IHC was noted on ≥5% of tumor cells in 71% of cHL (166/233) and in 54% (7/13) of NLPHL (22). In patients with cHL and normal 9p24.1 copy number, PD-L1 could still be overexpressed due to Epstein-Barr virus (EBV) infection (23). These data strongly suggest a potential genetic dependence upon PD-1 signaling in cHL.
PD-1 antibody monotherapy in HL has demonstrated high and durable response rates in early clinical studies (Table 1). The phase I study of nivolumab in HL (NCT01592370) showed an 87% objective response rate, with 17% reaching CR and 70% achieving PR (28). The phase II CheckMate 205 study (NCT02181738) of nivolumab in patients with relapsed/refractory (R/R) cHL after failed autologous stem cell transplant (ASCT) and brentuximab vedotin demonstrated an objective response rate of 66%% (53/80 patients, 95% CI: 54.8–76.4%), with 7 patients achieving a complete remission (9%) and 46 patients reaching a partial remission (58%) (30). The median duration of response was 8.7 months, with a median time to response of 2.1 months (range: 0.7–5.7 months) (28,30). High-level alterations of 9p24.1 and increased PD-L1 expression, although previously shown to be linked with chemoresistance and inferior outcomes in cHL, appear to be associated with more favorable responses to treatment with nivolumab (6,30).
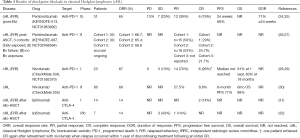
Full table
Pembrolizumab has also shown significant efficacy in R/R cHL patients as well. Updated results from KEYNOTE-013 (n=31) with a median follow-up of 24.9 months showed ORR 58% (18/31), CR 19% (6/31), and 12% achieving PR (12/31), with median duration of response not yet reached (25). In the phase II KEYNOTE-087 study (n=205), pembrolizumab was evaluated in 3 cohorts of cHL patients defined by history of exposure to brentuximab vedotin and ASCT. Pooled preliminary data from the three groups showed an ORR of 65.4–68.3%, CRR of 21.7–29%, and 93.7% had reduced tumor burden. The most common treatment related AEs were pyrexia (11%), hypothyroidism (10.5%), diarrhea (6.7%), fatigue (6.7%), headache (6.2%) rash (6.2%) and nausea (5.7%). This preliminary study shows significant clinical activity of pembrolizumab in all three cohorts, including chemo-refractory patients with cHL.
Long term follow-up safety data showing acute GVHD in 82% (14/17) of cHL patients treated with nivolumab who went on to allogeneic hematopoietic stem cell transplant (allo-HSCT) after participation in CheckMate 039 (n=5) and CheckMate 205 (n=12) suggest anti-PD-1 exposure prior to allo-HSCT may amplify risk of immune related complications after allogeneic transplantation. Grade 2–4 GVHD was seen in 10/17 (59%) and grade 3–4 in 5/17 (29%), with median time to onset of GVHD of 22 days. Two patients had hyperacute GVHD ≤14 days after transplant, and one patient with hepatic veno-occlusive disease died from multi-organ GVHD. Although numbers are small, these findings warrant studies of larger cohorts of patients with longer follow-up periods to understand risk and etiology of GVHD in patients who go on to allo-HSCT after PD-1 blockade (33).
In sum, these results led to accelerated FDA approval for nivolumab in cHL refractory to ASCT and brentuximab in May 2016 contingent upon a confirmatory phase III study. Results from a regulatory agency review of pembrolizumab data in cHL are expected soon. Practitioners are cautioned about allogeneic transplant following PD-1 pathway blockade due possible signal of increased risk of GVHD in this setting.
Other phase I and II clinical trials are currently underway in cHL comparing regimens with combinations of nivolumab with brentuximab (NCT02572167), nivolumab, ipilimumab, and brentuximab (NCT01896999) and nivolumab with ibrutinib (NCT02940301). Preliminary results from the CheckMate039 study of nivolumab plus ipilimumab were presented at ASH 2016, with an ORR in HL of 74% (n=23/31), with 6/31 reaching CR (19%), and 17 achieving PR (55%) (34). Preliminary response data from the ECOG-ACRIN E4412 study of combination therapy with brentuximab, ipilimumab, and nivolumab was also presented at ASH 2016, with an observed ORR of 100% in evaluable patients receiving brentuximab plus nivolumab, with CR rate of 62.5% (5/8), with 2 patients with prior brentuximab exposure achieving CR (35). There are also data exploring novel combinations of PD-1 agents with epigenetic modifiers in patients with refractory cHL (36). Clinical development of anti-PD-1 therapy in cHL continues, with a planned phase III of nivolumab monotherapy for cHL, and a phase III trial comparing pembrolizumab head-to-head with brentuximab (KEYNOTE-204, NCT02684292) as well as studies evaluating treatment with PD-1 blockade earlier in the natural history of cHL.
PD-1 blockade in non-Hodgkin lymphoma (NHL)
PD-L1 expression was found to be abundant in aggressive B-cell lymphoma, viral-associated lymphomas, and immunodeficiency-related lymphomas (37). Similar to cHL, primary mediastinal B cell lymphoma (PMBL), T-cell/histiocyte rich large B cell lymphoma, EBV+ DLBCLs such as DLBCL of the elderly and EBV+ immunodeficiency-associated lymphomas had 90–100% PD-L1/L2 expression driven by 9p24.1 gene amplification (38,39). Other subtypes of lymphoma noted to have PD-L1 expression include extranodal NK/T-cell lymphoma (83%), primary effusion lymphoma (75%), EBV(+) post-transplant lymphoproliferative disorder (PTLD, 70%), EBV(−) PTLD (57%), plasmablastic lymphoma (44%), and DLBCL-NOS (14%) (38). Primary testicular lymphoma, primary CNS lymphoma, mediastinal gray zone lymphoma and some T-cell lymphomas also have been reported to have 9p24.1 gene amplification and related PD-L1/PD-L2 overexpression (38). In addition to 9p24.1 amplification, there have also been translocations identified involving PD-L1 and PD-L2 in primary testicular lymphoma and primary CNS lymphoma (40).
In follicular lymphoma (FL), PD-1 expression in CD4+ tumor infiltrating lymphocytes (TILs) is associated with unresponsiveness to cytokines, a state consistent with T cell exhaustion (41). However, peripheral T cells and PD1− T cells exhibited normal activation upon exposure to cytokines (41). Although FL cells do not express PD1 ligands, histiocytes in the TME in FL do express PDL1, and suggest a potential rationale for use of anti-PD1 antibody in FL (41). Although PD1 is typically used to define exhausted T cells, it is highly expressed in T follicular helper cells, and has differential expression among exhausted T cells (42). Additional markers of T-cell exhaustion such as TIM-3 and LAG-3 are co-expressed with PD-1 in exhausted T cells, and represent potential targets for combination immunotherapy that can reverse T cell exhaustion, and as proof of concept that may be explored in future clinical trials, reversal of T cell exhaustion signaling has been demonstrated in vitro with anti-PD-1 and anti-LAG-3 (42,43).
Clinical evaluation of PD-1/PD-L1 blockade in NHL has been limited to phase I studies inclusive of multiple types of HM and some phase II studies demonstrating activity in DLBCL and FL (Table 2).
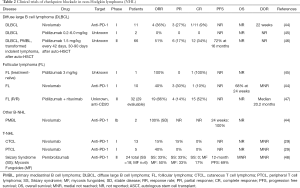
Full table
Pidilizumab, a humanized IgG1 monoclonal antibody intended to block PD-1, was evaluated in a phase II study of 66 patients with DLBCL after ASCT. This study had an ORR of 51%, with 70% of patients without PD at 16 months (46). A study of pidilizumab plus rituximab in 32 patients with relapsed FL demonstrated an ORR of 66% (19/29 evaluable patients) and 15 CRs were noted (52%) (47). Pidilizumab’s clinical development has been delayed by doubts about its target, as it does not bind PD-1 (49). Nivolumab monotherapy in FL showed a 40% ORR (4/10), with 1 CR (n=1, 10%), 3 PR (n=3, 30%), and 6 with stable disease (n=6, 60%), with median PFS not reached (NCT01592370) (44). The KEYNOTE-013 study included 19 patients with PMBL where pembrolizumab showed a response rate of 41%, with 2 patients achieving CR and 5 achieving PR (50). A phase II study (KEYNOTE-170) is planned based on these results.
In T cell NHL (T-NHL), pembrolizumab has shown clinical activity in advanced stage R/R mycosis fungoides (MF) and Sézary syndrome (SS), with ORR of 38% with 1 CR and 8 PRs (48). The CheckMate 039 study included 23 patients with T-NHL treated with nivolumab monotherapy in which there were 4 PRs, 2/13 in MF and 2/5 in peripheral T cell lymphoma (PTCL) (44). A recent series demonstrated a high rate of response to pembrolizumab in NK/T cell lymphoma (51). Multiple studies of immune checkpoint blockade are ongoing in several subtypes of NHL (Table 3).
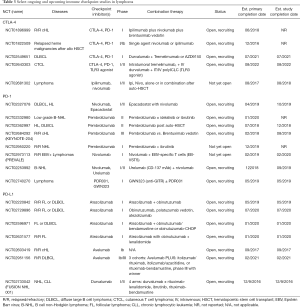
Full table
Varying levels of PD-1/PD-L1 expression in the TME are noted in NHL (37). Evaluation of PD-L1 expression in a small series of patients with aggressive B-cell NHL found that 3/7 responders (2 CRs, 1 PR) had high PD-L1 expression (30–100%) (52). Thus, in these preliminary analyses, PD-L1 IHC does not appear to consistently predict for responses.
Blockade of immune checkpoints in plasma cell myeloma
The myeloma TME
Multiple myeloma (MM) is a complex malignancy arising from plasma cells located within the bone marrow with known humoral and cellular immunodeficiency. MM is characterized by clonally heterogeneous malignant plasma cell populations that proliferate and persist in the bone marrow TME. The MM TME is comprised of osteoblasts, osteoclasts, bone marrow stromal cells (53), an immunosuppressive milieu of cytokines (54-58), myeloid-derived suppressor cells (MDSC) (59,60), regulatory T cells (61-63), and active PD-1/PD-L1 signaling, all of which contribute to local immune dysfunction and dysregulation.
In addition to the role played by other immune cells, MM cells directly contribute to cellular immune dysfunction. MM cells express HLA class II and may participate in cross-presentation of antigens and induction of immune tolerance to tumor antigens (64). MM cells can also express PD-L1, whereas normal plasma cells do not (65). PD-L1 expression on MM cells is associated with reduced susceptibility to cytotoxic effector T cell killing (65). PD-L1/PD-L2 expression in MM cells is driven by IFN-γ, toll-like receptor (TLR), Akt, and Ras signaling (65,66). Global defects in innate and adaptive immunity in myeloma include B cell dysfunction (hypogammaglobulinemia), abnormal dendritic cell (DC) number and function (67), natural killer (NK) (68-70), natural killer T-cell (NKT) (71), and T cell dysfunction (71,72). T cells in MM patients have been shown to have reduced cytotoxicity (73) and responsiveness to interleukin 2 (IL-2) (74), with alteration of the quantity and distribution of T cell subsets (63,75,76). APC in MM are also abnormal; DC isolated from myeloma patients have been shown to be functionally impaired (67). Plasmacytoid dendritic cells (pDC) are increased in the MM BM TME compared with healthy controls, and these cells are less able to trigger T cell proliferation (77). Despite multifactorial local immunosuppression in the MM TME, marrow-infiltrating T cells isolated from the MM TME retain the capacity to develop specific anti-MM immunity, demonstrated through ex vivo priming of T cells by DC that have processed tumor cell antigen outside the confines of the local MM TME (78).
In preclinical studies, syngeneic mice lacking PD-1 completely suppress growth of a MM tumor cell line (J558L), whereas mice expressing PD-1 rapidly develop tumor (79), suggesting a potential role for PD-1 blockade in treatment of myeloma. In the 5T33 model of myeloma, use of an anti-PD-L1 antibody in combination with lymphodepletion with radiation and a vaccine led to anti-myeloma activity (80). This effect was abrogated by depletion of CD4 or CD8 T cells, indicating that presence and function of both T cell subsets are necessary for this effect (80,81).
Although preclinical data supports a rationale for PD-1 blockade, nivolumab monotherapy did not show clinical efficacy (44) (Table 4). However, given that T cells are indeed capable of recognizing and killing MM cells, exploration of potential combinations with drug partners that might synergize with immune checkpoint blockade through modulation of the TME is an area of active drug development in MM. Several classes of anti-myeloma drugs exert immunomodulatory effects upon the TME and may synergize with immune checkpoint blockade and represent rational partners for immunotherapy drug development in MM. Immunomodulatory drugs (IMiDs) such as lenalidomide, pomalidomide, and thalidomide enhance anti-myeloma cellular immunity by augmenting T cell responsiveness to APCs, polarizing T cells towards a Th1 phenotype (85), inhibiting proliferation and function of Tregs (86), down-regulating PD-L1, and augmenting NK cell function. Lenalidomide has been shown to synergize with PD-1/PD-L1 blockade to inhibit immune suppression mediated by MDSC and enhance NK cell cytotoxicity in MM (60,87), and these agents through their action on the immune system are rational partners for use with immune checkpoint inhibition in MM.

Full table
Monoclonal antibodies against CD38 have entered clinical use for myeloma with the FDA approval of daratumumab for relapsed MM and its role in the TME may provide rationale for use of anti-CD38 antibodies in combination with immune checkpoint blockade. CD38 has pleiotropic expression and effects, mediating T cell anergy and exhaustion, and drives immunosuppressive activity of MDSC and Tregs (88-90). Use of daratumumab depletes CD38+ MDSC and Tregs in the TME and leads to expansion and skewing of T cell repertoire in patients with MM (91), suggesting that daratumumab partnered with immune checkpoint blockade might further activate T cells and drive anti-myeloma immune responses.
NK cells play an important role in the immune defense against myeloma (92), and blockade of inhibitory KIR receptors on NK cells is under evaluation as a therapeutic strategy in myeloma. KIRs are cell surface receptors present on both NK cells and some T cell subsets that recognize MHC class I molecules and modulate cell-mediated cytotoxicity (93). MM cells express surface ligands that bind to inhibitory KIRs and drive NK cell dysfunction (94), and there are studies underway to evaluate the potential to block inhibitory KIR signaling and restore MM-directed NK cell cytotoxicity. IPH2101, a human IgG4 against KIR2D inhibitory receptor, showed no objective responses and stable disease in 34% of RRMM patients, with no significant toxicity (95), and showed no single-agent activity in a phase II study of patients with smoldering MM (96). IPH2101 was also evaluated in combination with lenalidomide in 15 patients with R/R MM, and led to 5 objective responses (≥ MR): 2 VGPRs, 3 PRs, 1 MR, and 6 patients had SD (NCT01217203) (97). Lirilumab is a second-generation anti-KIR monoclonal antibody now in development in combination with elotuzumab (NCT02252263) and in combination with nivolumab (NCT01592370) in patients with R/R MM and lymphomas.
Ongoing and future drug combinations in MM
Ongoing clinical trials of IMiDs combined with PD-1 pathway blockade have shown promising preliminary results. The phase I KEYNOTE-023 (NCT02036502) study of pembrolizumab plus lenalidomide and dexamethasone in relapsed MM showed an overall response rate of 76% in 17 evaluable patients, with 5 patients achieving a PR or better (56%) (82). A phase II study (NCT02289222) combining pembrolizumab, pomalidomide, and dexamethasone had an ORR of 56% (27/48), ORR 55% among patients double-refractory to both proteasome inhibitors and IMiDs, and ORR 33% among patients with high-risk cytogenetics (98). Pembrolizumab, pomalidomide, and dexamethasone evaluated in a phase II study enrolling 48 patients with R/R MM, among which the overall response rate was ≥PR in 27 of 48 patients (55%), including sCR (n=4, 8%), nCR (n=3, 6%), VGPR (n=6, 13%), PR (n=14, 29%), and 7 minimal responses (15%), stable disease (n=9, 19%), 2 with progressive disease and 3 patients were not evaluable for response (83). Interestingly, responses correlated with presence of bone marrow infiltrating CD8+ effector T cells [ASH 2016 oral presentation (83)]. A retrospective series also supports the activity of this combination in a heavily pretreated and pomalidomide-exposed population, with an ORR of 33%, with 89% of patients achieving clinical benefit (3 PR, 2 MR, 3 SD) (84).
Building on the preliminary results of studies showing efficacy of approaches combining anti-myeloma drugs with immune checkpoint inhibitors discussed above (Table 4), several phase II and III clinical trials are planned and ongoing (Table 5). The phase III KEYNOTE-185 study of lenalidomide plus dexamethasone with or without pembrolizumab is planned (NCT02579863), and the phase III KEYNOTE-183 study (NCT02576977) is accruing R/R MM patients to evaluate pembrolizumab with or without pomalidomide and dexamethasone. A cohort in the CheckMate 039 study (NCT01592370) is currently enrolling patients to evaluate nivolumab plus daratumumab vs. nivolumab plus daratumumab, pomalidomide, and dexamethasone in R/R MM. Nivolumab, elotuzumab, pomalidomide, and dexamethasone will be evaluated in CheckMate 602 (NCT02726581). There are also trials underway in smoldering MM (pembrolizumab, NCT02603887; nivolumab plus lenalidomide and dexamethasone, NCT02903381). Studies are enrolling patients for treatment with ipilimumab plus nivolumab after ASCT (NCT02681302) and after allo-HSCT (NCT 01822509). A study evaluating durvalumab (anti-PD-L1) plus tremelimumab (anti-CTLA-4) after ASCT is also underway (NCT02716805).
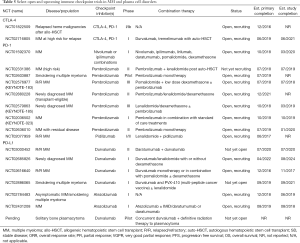
Full table
In summary, combination approaches using immune checkpoint inhibitors and anti-myeloma drugs has shown activity in preliminary results from several clinical trials, and studies of multiple combinations of anti-myeloma agents and immune active compounds are planned or underway.
Checkpoint blockade in chronic lymphocytic leukemia (CLL), acute myeloid leukemia (AML), and myelodysplasia
Although the vast majority of clinical data using checkpoint blockade in HM thus far has been in lymphoid cancers, a significant body of preclinical work supports exploration of the value of immune checkpoint inhibition in myeloid disorders. CTLA-4 is expressed on the malignant cell surface and in the cytoplasm in most patients with AML (99), chronic myeloid leukemia (CML), and CLL (100). Patients with AML with the CTLA-4 CT60 AA genotype have worse outcomes and higher rates of relapse after induction therapy in the first complete remission, suggesting potential impaired immune control of minimal residual disease after induction therapy (99). The PD-1 pathway plays a role in immune escape in CML (101). PD-1 is expressed on CLL cells in higher levels than in healthy controls, but PD-1 expression levels did not carry prognostic value in CLL (102). PD-L1 expression on CLL cells has been associated with impaired function of the immune synapse with T cells (103).
In the myelodysplastic syndrome (MDS), there is evidence that PD-1 pathway blockade may be a promising avenue for treatment. PD-L1 is expressed at higher levels on blasts in patients with high risk MDS and more refractory disease. Additionally, there are data that azacitidine upregulates PD-1 and PD-L1 in MDS and that this is associated with emergence of resistance to azacitidine (104).
Thus far, clinical experience using immune checkpoint blockade in patients with leukemia is limited to early phase clinical trials (Table 6). In a phase I study of pidilizumab, 8 patients with AML were included, of which 7 of 8 had no change in average percentage of blasts in peripheral blood at 21 days, and one of 8 patients had a response, with peripheral blasts percentage dropping to 5% from 50%, and who ultimately had disease progression 61 weeks after receiving pidilizumab (45). In patients with previously treated or untreated MDS, a phase II study is ongoing evaluating the combination of nivolumab or ipilimumab with 5-azacitidine study (105). Preliminary results showed that single agent ipilimumab is capable of inducing responses in previously treated MDS patients (ORR 22%), however, single agent nivolumab showed no clinical activity (105). Azacitidine plus nivolumab had an ORR of 69% (9/13) in those patients who previously failed azacitidine treatment (105). Early phase studies evaluating ipilimumab in pan-HM with relapse after allo-HSCT have shown a low rate of response in lymphoma, with some responses seen in patients with myeloid disorders. Bashey et al. carried out a phase I study of ipilimumab in patients with recurrent or progressive HM after allogeneic stem cell transplant, enrolling 29 patients and evaluating for safety and efficacy as the primary outcome (31). Patients were treated with ipilimumab 10 mg/kg every 3 weeks, and notably 4 of 5 patients with extramedullary AML involving the skin (leukemia cutis) achieved a durable CR lasting more than 1 year (31). In the post-allo-HSCT setting, there has not been significant evidence of induction or worsening of graft versus host disease (GVHD) (31,32). Six patients in this study patients (21%) had immune-related adverse events (irAE), 4 patients (14%) had GVHD precluding further use of ipilimumab, and there was one death attributable to therapy (32). Based on these early results, CTLA-4 blockade after allogeneic stem cell transplant is undergoing additional study. Secondary endpoint data analysis showed CTLA-4 blockade by a single infusion of ipilimumab increased CD4+ and CD4+/HLA-DR+ T lymphocyte counts and augmented intracellular CTLA-4 expression at the highest dose level. There was no significant change in Treg cell numbers after ipilimumab infusion (106).
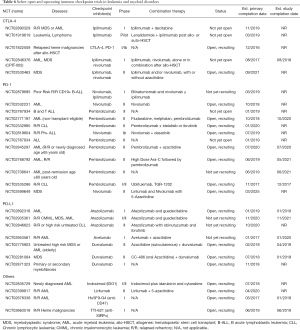
Full table
Current ongoing studies target relapsed leukemia patient population and evaluate safety and effect of single immune checkpoint inhibitor use (nivolumab and ipilimumab), single versus combined immune checkpoint inhibitor use, novel combinations using checkpoint antibodies with other immunotherapeutic approaches such as the engineered bi-specific antibody (BiTE) against CD3 and CD19 (blinatumomab), and combined use of epigenetic therapies with immune checkpoint inhibitors (decitabine and ipilimumab) (Table 6).
Immune-related adverse events and the checkpoint inhibitors
The phase I studies of nivolumab, pembrolizumab and pidilizumab demonstrate a favorable safety profile of these agents, with rates of drug related grade 3 adverse events ranging from 18–20% (28,44,45,107). IrAE were common and typically lower grade. There were 13/134 (9.7%) cases of pneumonitis, with three severe (grade 4) and one fatal case of pneumonitis observed (25,44-46,107). Although pulmonary toxicity is a known complication associated with treatment with PD-1 inhibitors (108), it is important to note in a patient population treated with agents with known potential for pulmonary toxicity such as radiation, carmustine, lenalidomide, pomalidomide, and bleomycin, there was not an excessive rate of pneumonitis noted in the phase I studies of PD-1 agents (109). Early data in melanoma suggests gut microbiota may play a role in development of colitis, however for the most part predictors of toxicity from immune checkpoint inhibitors are lacking (110).
Mechanisms of resistance to immune checkpoint blockade
Although a subset of patients with HM obtain benefit from treatment with immune checkpoints, mechanisms of resistance to these therapies remain poorly understood. Emerging data suggest alterations in MHC class I in the TME may limit tumors’ responsiveness to immune-based approaches. Of note, a recent cohort study found that 75% (40/53) patients with DLBCL commonly fails to express HLA class I (111), and β2M mutations and deletions, and abnormalities in CD58 (a molecule involved in T cell and NK cell signaling), led to a lack of membrane HLA-I expression (111). Effector T-cells require intact MHC to bind the TCR in order to exert cellular cytotoxicity against tumor cells. It is logical to consider mutations causing reduced MHC expression on the tumor cell surface may affect response to immune checkpoint blockade. Beta-2 microglobulin (β2M) is a required component for assembly and surface expression of MHC class I, and a retrospective series evaluated β2M, MHC I, and MHC II expression, and found decreased or absent expression of β2M and MHC I in 80% and decreased or absent MHC class II in 70% of cHL patients. Reduced β2M and MHC class I expression in cHL patients was associated with an inferior outcome independent of 9p24.1 status (112). The association of response to checkpoint blockade in patients with β2M, MHC class I, and MHC class II mutations have not yet been described in cHL. However, data from melanoma patients treated with PD-1 blockade demonstrating alterations in β2M and loss of MHC class I led to disease progression and resistance to PD-1 blockade immunotherapy (113), suggesting that efficacy of PD-1 blockade in cHL may be independent of antigen presentation by the malignant cell. Other mechanisms of resistance to immune checkpoint blockade, such as T cell exhaustion, inhibitory factors in the TME such as ADO and IDO, and novel mutations that mediate resistance remain active areas of investigation (114,115).
Novel immune checkpoints and combination therapies
Alternative immune cell co-receptors and targets in the immune microenvironment (beyond PD-1/PD-L1 and CTLA-4) represent potential opportunities for immunotherapy drug development and are in preclinical development or early phase clinical trials (Table 7) and are reviewed extensively elsewhere (2). In addition to blockade of immune checkpoints relevant to T-cell function, blockade of CD47, an innate immune checkpoint that inhibits phagocytosis by macrophages, has shown activity in multiple preclinical tumor models (116-122) as a potential drug target and several agents are in phase I clinical trials enrolling patients with HM (123,124). There is also intense interest in combinatorial approaches using cancer vaccines, chemotherapy, and radiation with immune checkpoint blockade to expand efficacy and response rates to checkpoint blockade, and novel clinical trials are underway to evaluate these approaches (125).
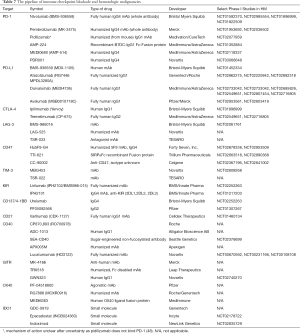
Full table
Conclusions
Development of immune checkpoint inhibitors is an important advance in the treatment of human cancers, and this approach has shown significant promise for treatment of a variety of HM. Novel drug combinations, identification of targets in the immune TME, and clinical trials of agents that modulate immune checkpoints beyond CTLA-4 and PD-1/PD-L1 are ongoing with the aim to expand the utility of immune-based approaches for treatment of all patients with lymphoid, plasma cell and myeloid cancers. Ongoing attention to toxicity from immune-based approaches such as increased rates of GVHD in patients who go on to receive allogeneic stem cell transplantation after checkpoint blockade remains quite important. Clinical trials and detailed correlative studies evaluating T cell response, TME, and host factors will hopefully facilitate an understanding of how to gain durable disease control from immunotherapy while minimizing the risk of immune-related toxicities.
Acknowledgements
The authors would like to thank the patients and their families who have participated and are participating in clinical trials and advancing care for patients everywhere.
Funding: This work was supported in part by the Memorial Sloan Kettering Cancer Center (MSKCC) NCI core grant P30 CA008748 (AM Lesokhin), the Mortimer J. Lacher Fellowship established by The Lymphoma Foundation (MJ Pianko), and is also supported in part by a grant from the National Institutes of Health/National Center for Advancing Translational Sciences (UL1TR00457), administered by the Clinical and Translational Science Center at Weill Cornell Medical Center and MSKCC. AM Lesokhin is a member of the Parker Institute for Cancer Immunotherapy, which supported the MSKCC Cancer Immunotherapy Program. AM Lesokhin also receives support from the Memorial Sloan Kettering Sawiris Foundation and MSKCC Cycle for Survival.
Footnote
Conflicts of Interest: AM Lesokhin: Stock or other ownership: Exelixis, Enumeral; Honoraria: Bristol-Myers Squibb, Janssen Pharmaceuticals (a Johnson & Johnson Co.), Gilead Sciences (I), Novartis; Consulting or advisory role: Bristol-Myers Squibb, Foundation Medicine (Inst), Janssen Pharmaceuticals (a Johnson & Johnson Co.), Novartis, Juno, Aduro; Research funding: Bristol-Myers Squibb (Inst), Janssen Pharmaceuticals (a Johnson & Johnson Co.) (Inst); Patents, royalties, other intellectual property: Serametrix. The other authors have no conflicts of interest to declare.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Lesokhin AM, Callahan MK, Postow MA, et al. On being less tolerant: Enhanced cancer immunosurveillance enabled by targeting checkpoints and agonists of T cell activation. Sci Transl Med 2015;7:280sr1. [Crossref] [PubMed]
- Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994;1:405-13. [Crossref] [PubMed]
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008;8:467-77. [Crossref] [PubMed]
- Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest 2015;125:3384-91. [Crossref] [PubMed]
- Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J Clin Oncol 2016;34:2690-7. [Crossref] [PubMed]
- Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016;17:e542-51. [Crossref] [PubMed]
- Wolchok JD, Weber JS, Maio M, et al. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol 2013;24:2174-80. [Crossref] [PubMed]
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010;11:155-64. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2980-7. [Crossref] [PubMed]
- Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016;17:1558-68. [Crossref] [PubMed]
- Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:1270-1. [Crossref] [PubMed]
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908-18. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Dang TO, Ogunniyi A, Barbee MS, et al. Pembrolizumab for the treatment of PD-L1 positive advanced or metastatic non-small cell lung cancer. Expert Rev Anticancer Ther 2016;16:13-20. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol 2016;34:3119-25. [Crossref] [PubMed]
- Levy A, Massard C, Soria JC, et al. Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: Single centre subset analysis from a phase 1/2 trial. Eur J Cancer 2016;68:156-62. [Crossref] [PubMed]
- Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010;116:3268-77. [Crossref] [PubMed]
- Menter T, Bodmer-Haecki A, Dirnhofer S, et al. Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum Pathol 2016;54:17-24. [Crossref] [PubMed]
- Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res 2012;18:1611-8. [Crossref] [PubMed]
- Zinzani PL, Ribrag V, Moskowitz CH, et al. Phase 1b Study of PD-1 Blockade with Pembrolizumab in Patients with Relapsed/Refractory Primary Mediastinal Large B-Cell Lymphoma (PMBCL). Blood 2015;126:3986.
- Armand P, Shipp M, Ribrag V, et al. Pembrolizumab in Patients with Classical Hodgkin Lymphoma after Brentuximab Vedotin Failure: Long-Term Efficacy from the Phase 1b Keynote-013 Study. Blood 2016;128:1108.
- Chen RW, Zinzani PL, Fanale MA, et al. Pembrolizumab for relapsed/refractory classical Hodgkin lymphoma (R/R cHL): phase 2 KEYNOTE-087 study. J Clin Oncol 2016;34:abstr 7555.
- Moskowitz CH, Zinzani P, Fanale M, et al. Pembrolizumab in Relapsed/Refractory Classical Hodgkin Lymphoma: Primary End Point Analysis of the Phase 2 Keynote-087 Study. Blood 2016;128:1107.
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311-9. [Crossref] [PubMed]
- Ansell S, Armand P, Timmerman JM, et al. Nivolumab in Patients (Pts) with Relapsed or Refractory Classical Hodgkin Lymphoma (R/R cHL): Clinical Outcomes from Extended Follow-up of a Phase 1 Study (CA209-039). Blood 2015;126:583.
- Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 2016;17:1283-94. [Crossref] [PubMed]
- Bashey A, Medina B, Corringham S, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood 2009;113:1581-8. [Crossref] [PubMed]
- Davids MS, Kim HT, Bachireddy P, et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N Engl J Med 2016;375:143-53. [Crossref] [PubMed]
- Armand P, Zinzani P, Collins GP, et al. Outcomes of Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) after Treatment with Nivolumab for Relapsed/Refractory Hodgkin Lymphoma. Blood 2016;128:3502.
- Ansell S, Gutierrez ME, Shipp M, et al. A Phase 1 Study of Nivolumab in Combination with Ipilimumab for Relapsed or Refractory Hematologic Malignancies (CheckMate 039). Blood 2016;128:183.
- Diefenbach CS, Hong F, David KA, et al. A Phase I Study with an Expansion Cohort of the Combination of Ipilimumab and Nivolumab and Brentuximab Vedotin in Patients with Relapsed/Refractory Hodgkin Lymphoma: A Trial of the ECOG-ACRIN Cancer Research Group (E4412 Arms D and E). Blood 2016;128:1106.
- Falchi L, Sawas A, Deng C, et al. PD-1 Blockade after Epigenetic Therapy in Patients with Relapsed or Refractory Hodgkin Lymphoma: Higher-Than-Expected Rate of Complete Responses. Blood 2016;128:2999.
- Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 2013;19:3462-73. [Crossref] [PubMed]
- Hutchinson CB, Wang E. Primary mediastinal (thymic) large B-cell lymphoma: a short review with brief discussion of mediastinal gray zone lymphoma. Arch Pathol Lab Med 2011;135:394-8. [PubMed]
- Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med 2003;198:851-62. [Crossref] [PubMed]
- Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood 2016;127:869-81. [Crossref] [PubMed]
- Myklebust JH, Irish JM, Brody J, et al. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood 2013;121:1367-76. [Crossref] [PubMed]
- Yang ZZ, Price-Troska T, Novak AJ, et al. The Exhausted Intratumoral T Cell Population in B-Cell Non-Hodgkin Lymphoma Is Defined By LAG-3, PD-1 and Tim-3 Expression. Blood 2015;126:2661. [PubMed]
- Yang ZZ, Grote DM, Ziesmer SC, et al. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J 2015;5:e281. [Crossref] [PubMed]
- Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J Clin Oncol 2016;34:2698-704. [Crossref] [PubMed]
- Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 2008;14:3044-51. [Crossref] [PubMed]
- Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol 2013;31:4199-206. [Crossref] [PubMed]
- Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol 2014;15:69-77. [Crossref] [PubMed]
- Khodadoust M, Rook AH, Porcu P, et al. Pembrolizumab for Treatment of Relapsed/Refractory Mycosis Fungoides and Sezary Syndrome: Clinical Efficacy in a Citn Multicenter Phase 2 Study. Blood 2016;128:181.
- Carroll J. Anti-PD-1? Well, no, says Medivation as a partial hold forces a halt to 'pivotal' cancer study 2016 [updated 01/26/2016]. Available online: http://www.fiercebiotech.com/r-d/anti-pd-1-well-no-says-medivation-as-a-partial-hold-forces-a-halt-to-pivotal-cancer-study
- Zinzani P, Ribrag V, Moskowitz CH, et al. Phase 1b Study of Pembrolizumab in Patients with Relapsed/Refractory Primary Mediastinal Large B-Cell Lymphoma: Results from the Ongoing Keynote-013 Trial. Blood 2016;128:619.
- Kwong YL, Chan TS, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing L-asparaginase. Blood 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Bohl SR, Schoensteiner S, Huber H, et al. Nivolumab Induces Remission in High- PD-L1 Expressing Aggressive B-Non Hodgkin Lymphoma: A Single Center Experience. Blood 2016;128:1865.
- Gorgun G, Anderson KC. Intrinsic modulation of lymphocyte function by stromal cell network: advance in therapeutic targeting of cancer. Immunotherapy 2011;3:1253-64. [Crossref] [PubMed]
- Cook G, Campbell JD, Carr CE, et al. Transforming growth factor beta from multiple myeloma cells inhibits proliferation and IL-2 responsiveness in T lymphocytes. J Leukoc Biol 1999;66:981-8. [PubMed]
- Filella X, Blade J, Guillermo AL, et al. Cytokines (IL-6, TNF-alpha, IL-1alpha) and soluble interleukin-2 receptor as serum tumor markers in multiple myeloma. Cancer Detect Prev 1996;20:52-6. [PubMed]
- Brimnes MK, Svane IM, Johnsen HE. Impaired functionality and phenotypic profile of dendritic cells from patients with multiple myeloma. Clin Exp Immunol 2006;144:76-84. [Crossref] [PubMed]
- Lu ZY, Zhang XG, Rodriguez C, et al. Interleukin-10 is a proliferation factor but not a differentiation factor for human myeloma cells. Blood 1995;85:2521-7. [PubMed]
- Caux C, Massacrier C, Vanbervliet B, et al. Interleukin 10 inhibits T cell alloreaction induced by human dendritic cells. Int Immunol 1994;6:1177-85. [Crossref] [PubMed]
- Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 2006;203:2691-702. [Crossref] [PubMed]
- Gorgun GT, Whitehill G, Anderson JL, et al. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood 2013;121:2975-87. [Crossref] [PubMed]
- Beyer M, Kochanek M, Giese T, et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood 2006;107:3940-9. [Crossref] [PubMed]
- Feyler S, von Lilienfeld-Toal M, Jarmin S, et al. CD4(+)CD25(+)FoxP3(+) regulatory T cells are increased whilst CD3(+)CD4(-)CD8(-)alphabetaTCR(+) Double Negative T cells are decreased in the peripheral blood of patients with multiple myeloma which correlates with disease burden. Br J Haematol 2009;144:686-95. [Crossref] [PubMed]
- Mills KH, Cawley JC. Abnormal monoclonal antibody-defined helper/suppressor T-cell subpopulations in multiple myeloma: relationship to treatment and clinical stage. Br J Haematol 1983;53:271-5. [Crossref] [PubMed]
- Sotomayor EM, Borrello I, Rattis FM, et al. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood 2001;98:1070-7. [Crossref] [PubMed]
- Liu J, Hamrouni A, Wolowiec D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood 2007;110:296-304. [Crossref] [PubMed]
- Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal 2012;5:ra46. [Crossref] [PubMed]
- Brown RD, Pope B, Murray A, et al. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood 2001;98:2992-8. [Crossref] [PubMed]
- Jurisic V, Srdic T, Konjevic G, et al. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med Oncol 2007;24:312-7. [Crossref] [PubMed]
- Konjevic G, Vuletic A, Mirjacic Martinovic K, et al. Decreased CD161 activating and increased CD158a inhibitory receptor expression on NK cells underlies impaired NK cell cytotoxicity in patients with multiple myeloma. J Clin Pathol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Dosani T, Carlsten M, Maric I, et al. The cellular immune system in myelomagenesis: NK cells and T cells in the development of myeloma [corrected] and their uses in immunotherapies. Blood Cancer J 2015;5:e306. [Crossref] [PubMed]
- Dhodapkar MV, Geller MD, Chang DH, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med 2003;197:1667-76. [Crossref] [PubMed]
- Atanackovic D, Luetkens T, Kroger N. Coinhibitory molecule PD-1 as a potential target for the immunotherapy of multiple myeloma. Leukemia 2014;28:993-1000. [Crossref] [PubMed]
- Massaia M, Dianzani U, Bianchi A, et al. Defective generation of alloreactive cytotoxic T lymphocytes (CTL) in human monoclonal gammopathies. Clin Exp Immunol 1988;73:214-8. [PubMed]
- Rutella S, Locatelli F. Targeting multiple-myeloma-induced immune dysfunction to improve immunotherapy outcomes. Clin Dev Immunol 2012;2012:196063. [Crossref]
- Pratt G, Goodyear O, Moss P. Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol 2007;138:563-79. [Crossref] [PubMed]
- Schutt P, Brandhorst D, Stellberg W, et al. Immune parameters in multiple myeloma patients: influence of treatment and correlation with opportunistic infections. Leuk Lymphoma 2006;47:1570-82. [Crossref] [PubMed]
- Chauhan D, Singh AV, Brahmandam M, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell 2009;16:309-23. [Crossref] [PubMed]
- Dhodapkar MV, Krasovsky J, Olson K. T cells from the tumor microenvironment of patients with progressive myeloma can generate strong, tumor-specific cytolytic responses to autologous, tumor-loaded dendritic cells. Proc Natl Acad Sci U S A 2002;99:13009-13. [Crossref] [PubMed]
- Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99:12293-7. [Crossref] [PubMed]
- Kearl TJ, Jing W, Gershan JA, et al. Programmed death receptor-1/programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myeloma. J Immunol 2013;190:5620-8. [Crossref] [PubMed]
- Chung DJ, Pronschinske KB, Shyer JA, et al. Immune Reconstitution after Autologous Stem Cell Transplantation for Multiple Myeloma. Biol Blood Marrow Transplant 2014;20:S57-71. [Crossref]
- San Miguel J, Mateos MV, Shah JJ, et al. Pembrolizumab in Combination with Lenalidomide and Low-Dose Dexamethasone for Relapsed/Refractory Multiple Myeloma (RRMM): Keynote-023. Blood 2015;126:505.
- Badros A, Hyjek E, Ma N, et al. Pembrolizumab in Combination with Pomalidomide and Dexamethasone for Relapsed/Refractory Multiple Myeloma (RRMM). American Society of Hematology Annual Meeting; December 4th, 2016. San Diego, CA: 2016;653.
- Wilson L, Cohen AD, Weiss B, et al. Pembrolizumab in Combination with Pomalidomide and Dexamethasone (PEMBRO/POM/DEX) for Pomalidomide Exposed Relapsed or Refractory Multiple Myeloma. Blood 2016;128:2119.
- Luptakova K, Rosenblatt J, Glotzbecker B, et al. Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol Immunother 2013;62:39-49. [Crossref] [PubMed]
- Galustian C, Meyer B, Labarthe MC, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother 2009;58:1033-45. [Crossref] [PubMed]
- Gorgun G, Samur MK, Cowens KB, et al. Lenalidomide Enhances Immune Checkpoint Blockade-Induced Immune Response in Multiple Myeloma. Clin Cancer Res 2015;21:4607-18. [Crossref] [PubMed]
- Leone RD, Lo YC, Powell JD. A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J 2015;13:265-72. [Crossref] [PubMed]
- Serra S, Horenstein AL, Vaisitti T, et al. CD73-generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug-induced cell death. Blood 2011;118:6141-52. [Crossref] [PubMed]
- Young A, Mittal D, Stagg J, et al. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov 2014;4:879-88. [Crossref] [PubMed]
- Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016;128:384-94. [Crossref] [PubMed]
- Godfrey J, Benson DM Jr. The role of natural killer cells in immunity against multiple myeloma. Leuk Lymphoma 2012;53:1666-76. [Crossref] [PubMed]
- Benson DM Jr, Caligiuri MA. Killer immunoglobulin-like receptors and tumor immunity. Cancer Immunol Res 2014;2:99-104. [Crossref] [PubMed]
- Carbone E, Neri P, Mesuraca M, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood 2005;105:251-8. [Crossref] [PubMed]
- Benson DM Jr, Hofmeister CC, Padmanabhan S, et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood 2012;120:4324-33. [Crossref] [PubMed]
- Korde N, Carlsten M, Lee MJ, et al. A phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica 2014;99:e81-3. [Crossref] [PubMed]
- Benson DM Jr, Cohen AD, Jagannath S, et al. A Phase I Trial of the Anti-KIR Antibody IPH2101 and Lenalidomide in Patients with Relapsed/Refractory Multiple Myeloma. Clin Cancer Res 2015;21:4055-61. [Crossref] [PubMed]
- Badros AZ, Kocoglu MH, Ma N, et al. A Phase II Study of Anti PD-1 Antibody Pembrolizumab, Pomalidomide and Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2015;126:506.
- Perez-Garcia A, Brunet S, Berlanga JJ, et al. CTLA-4 genotype and relapse incidence in patients with acute myeloid leukemia in first complete remission after induction chemotherapy. Leukemia 2009;23:486-91. [Crossref] [PubMed]
- Ciszak L, Frydecka I, Wolowiec D, et al. CTLA-4 affects expression of key cell cycle regulators of G0/G1 phase in neoplastic lymphocytes from patients with chronic lymphocytic leukaemia. Clin Exp Med 2016;16:317-32. [Crossref] [PubMed]
- Christiansson L, Soderlund S, Svensson E, et al. Increased level of myeloid-derived suppressor cells, programmed death receptor ligand 1/programmed death receptor 1, and soluble CD25 in Sokal high risk chronic myeloid leukemia. PLoS One 2013;8:e55818. [Crossref] [PubMed]
- Grzywnowicz M, Zaleska J, Mertens D, et al. Programmed death-1 and its ligand are novel immunotolerant molecules expressed on leukemic B cells in chronic lymphocytic leukemia. PLoS One 2012;7:e35178. [Crossref] [PubMed]
- Ramsay AG, Clear AJ, Fatah R, et al. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood 2012;120:1412-21. [Crossref] [PubMed]
- Yang H, Bueso-Ramos C, DiNardo C, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 2014;28:1280-8. [Crossref] [PubMed]
- Garcia-Manero G, Daver NG, Montalban-Bravo G, et al. A Phase II Study Evaluating the Combination of Nivolumab (Nivo) or Ipilimumab (Ipi) with Azacitidine in Pts with Previously Treated or Untreated Myelodysplastic Syndromes (MDS). Blood 2016;128:344.
- Zhou Q, Munger ME, Veenstra RG, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 2011;117:4501-10. [Crossref] [PubMed]
- Moskowitz CH, Ribrag V, Michot JM, et al. PD-1 Blockade with the Monoclonal Antibody Pembrolizumab (MK-3475) in Patients with Classical Hodgkin Lymphoma after Brentuximab Vedotin Failure: Preliminary Results from a Phase 1b Study (KEYNOTE-013). Blood 2014;124:290.
- Nishino M, Ramaiya NH, Awad MM, et al. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin Cancer Res 2016;22:6051-60. [Crossref] [PubMed]
- Armand P. Immune checkpoint blockade in hematologic malignancies. Blood 2015;125:3393-400. [Crossref] [PubMed]
- Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 2016;7:10391. [Crossref] [PubMed]
- Challa-Malladi M, Lieu YK, Califano O, et al. Combined genetic inactivation of beta2-Microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell 2011;20:728-40. [Crossref] [PubMed]
- Roemer MG, Advani RH, Redd RA, et al. Classical Hodgkin Lymphoma with Reduced beta2M/MHC Class I Expression is Associated with Inferior Outcome Independent of 9p24.1 Status. Cancer Immunol Res 2016;4:910-6. [Crossref] [PubMed]
- Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 2016;375:819-29. [Crossref] [PubMed]
- O'Donnell JS, Long GV, Scolyer RA, et al. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev 2017;52:71-81. [Crossref] [PubMed]
- O'Donnell JS, Smyth MJ, Teng MW. Acquired resistance to anti-PD1 therapy: checkmate to checkpoint blockade? Genome Med 2016;8:111. [Crossref] [PubMed]
- Jaiswal S, Jamieson CH, Pang WW, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009;138:271-85. [Crossref] [PubMed]
- Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009;138:286-99. [Crossref] [PubMed]
- Chao MP, Alizadeh AA, Tang C, et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res 2011;71:1374-84. [Crossref] [PubMed]
- Chao MP, Alizadeh AA, Tang C, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 2010;142:699-713. [Crossref] [PubMed]
- Chao MP, Tang C, Pachynski RK, et al. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood 2011;118:4890-901. [Crossref] [PubMed]
- Kim D, Wang J, Willingham SB, et al. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia 2012;26:2538-45. [Crossref] [PubMed]
- Tseng D, Volkmer JP, Willingham SB, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A 2013;110:11103-8. [Crossref] [PubMed]
- Liu J, Wang L, Zhao F, et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS One 2015;10:e0137345. [Crossref] [PubMed]
- Petrova PS, Viller NN, Wong M, et al. TTI-621 (SIRPαFc): A CD47-Blocking Innate Immune Checkpoint Inhibitor with Broad Antitumor Activity and Minimal Erythrocyte Binding. Clin Cancer Res 2017;23:1068-79. [Crossref] [PubMed]
- Hellmann MD, Friedman CF, Wolchok JD. Combinatorial Cancer Immunotherapies. Adv Immunol 2016;130:251-77. [Crossref] [PubMed]
Cite this article as: Pianko MJ, Liu Y, Bagchi S, Lesokhin AM. Immune checkpoint blockade for hematologic malignancies: a review. Stem Cell Investig 2017;4:32.


