De-liver CLiPs and revitalize hepatocytes
The liver is a highly quiescent organ, which has made the existence of hepatic stem cells debatable (1). However, upon injury, the liver shows a remarkable regenerative capacity unmatched by most human organs (2,3). Recent findings shed new light on the origins of liver progenitor cells (LPCs) and source of new hepatocytes during homeostasis and repair. Numerous groups have found that mature hepatocytes (MHs) themselves are the source of hepatic progenitor cells that contribute to liver regeneration (4,5). These findings highlight the plasticity of hepatocytes and a potential target for cell-based therapy in patients with severe liver dysfunction. If hepatocytes have that capacity in vivo, reprogramming of MHs in vitro might be a step in attaining liver regeneration.
Katsuda et al. 2016 recently developed a new method for exposing the plasticity of hepatocytes to de-differentiate them back into their progenitor state (6). They showed that a subset of MHs, particularly diploid MHs, are more susceptible to reprogramming compared to polyploid MHs. This is in line with findings by Wang et al., which showed in vivo, diploid hepatocytes residing around central veins have a higher proliferative capacity and characteristics of progenitor cells during the turnover of hepatic cells under homeostatic conditions (4). Katsuda et al. (2016) found that a mixture of three small molecules Y-27632, PD0325901, A-83-01, and CHIR99021 (YAC) can reprogram rat and mouse MHs into bipotential LPCs, in vitro, which the authors refer to as chemically induced liver progenitors (CLiPs) (Figure 1A). CLiPs were shown to differentiate into both MHs and biliary epithelial cells (BECs). In immunodeficient mice with CCl4 induced chronic liver injury, CLiPs had the capacity to differentiate into both MHs and BECs with a 75–90% efficiency. Highlighting, their capacity to function as bona fide LPCs. Furthermore, the morphology of YAC-induced proliferative cells was similar to oval cells due to their high nucleus/cytoplasm ratio, along with an increase in numerous LPC markers. YAC-induced proliferative cells exposed to factors for hepatic stimulation developed MH-like morphology and showed hepatocyte-like functionality in terms of Alb and Hnf4α expression, albumin secretion, glycogen storage, ability to induce Cyp1a activity, and urea synthesis. This was further confirmed through mRNA Microarray analysis of hepatocytes derived from the CLiP method, which shows genes associated with hepatic functions found in MHs. BEC were also developed through YAC treatment of MHs, and had higher expression of genes CK19 and Grhl2 than un-induced cells. Up-regulation of aquaporin genes Aqp1 and Aqp9 along with ion channel genes Cftr and Ae2 further suggests that these are functional ductal cells. Prominently, LPCs derived from CLiP technology can be expanded safely in vivo and cease proliferation, returning to a quiescent state after CCl4 induced injury. In vitro, the authors showed that YAC enhanced the proliferative capacity of MHs over a 2-week culture by approximately 29.8±8.0 times. This is an extraordinary achievement in enhancing proliferation of cells that may potentially be used for tissue/liver engineering. The proliferation of cells subjected to YAC underwent re-differentiation into MHs under hepatocyte inducible conditions without exhibiting tumorigenic properties. CLiPs have been shown to be expanded efficiently in vitro with short doubling time and a high efficiency in replacing injured hepatic cells. The authors showed that the proliferative capacity of CLiPs is unaffected even after 26 passages with a doubling time of 14.7±1.1 hrs. This raises the possibility that CLiPSs might be oncogenic, albeit removal of YAC resulted in a drastic loss of proliferative capacity. Arguably, this highlights the potent role of YAC in regulating proliferation of CLiPs and how it’s not the cells themselves that become oncogenic. This was further analyzed through Sanger sequences for p53, p21, and Kras, which showed no mutations of these markers in CLiPs. It should be noted that chromosomal abnormalities in 2 out 3 CLiP lines presents a significant hurdle in getting this to patients. Thus, consistency in chromosomal stability is an essential next step. Furthermore, CLiP derived cells would still need to be screened for genetic abnormalities, not limited to few genes, due to karyotypic instability, given their very rapid proliferative capacity.
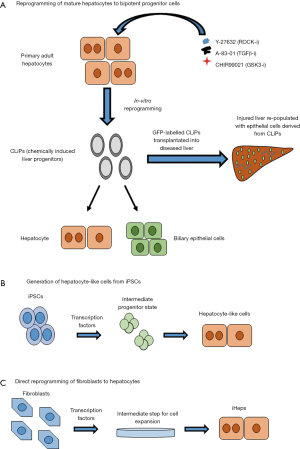
There is a great unmet medical need for liver therapeutics that would relieve the demand for transplants. The field is moving towards cell-based therapies that can replace damaged hepatic cells (7). Recent progress with induced pluripotent stem cells (Figure 1B) provides some hope; however, these cells are more fetal in their characteristics and don’t achieve the full functional capacity of MHs and still need to be evaluated for tumorigenicity (8). Other groups have done direct reprogramming of fibroblasts to hepatocytes (Figure 1C) showing comparable gene expression to MHs but not identical (9). Arguably, prior to clinical application, these techniques should be tested and proven successful in large animal models, as they are better predictors of responses in humans than are rodents (10).
Acknowledgements
We thank Toronto Hydro for their generous donation to our research.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sadri AR, Jeschke MG, Amini-Nik S. Advances in Liver Regeneration: Revisiting Hepatic Stem/Progenitor Cells and Their Origin. Stem Cells Int 2016;2016:7920897. [Crossref] [PubMed]
- Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med 2014;20:857-69. [Crossref] [PubMed]
- Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci 2013;70:2059-81. [Crossref] [PubMed]
- Wang B, Zhao L, Fish M, et al. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature 2015;524:180-5. [Crossref] [PubMed]
- Font-Burgada J, Shalapour S, Ramaswamy S, et al. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell 2015;162:766-79. [Crossref] [PubMed]
- Katsuda T, Kawamata M, Hagiwara K, et al. Conversion of Terminally Committed Hepatocytes to Culturable Bipotent Progenitor Cells with Regenerative Capacity. Cell Stem Cell 2017;20:41-55. [Crossref] [PubMed]
- Forbes SJ, Gupta S, Dhawan A. Cell therapy for liver disease: From liver transplantation to cell factory. J Hepatol 2015;62:S157-69. [Crossref] [PubMed]
- Baxter M, Withey S, Harrison S, et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J Hepatol 2015;62:581-9. [Crossref] [PubMed]
- Huang P, Zhang L, Gao Y, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell 2014;14:370-84. [Crossref] [PubMed]
- Forbes SJ, Newsome PN. Liver regeneration - mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol 2016;13:473-85. [Crossref] [PubMed]
Cite this article as: Sadri AR, Amini-Nik S. De-liver CLiPs and revitalize hepatocytes. Stem Cell Investig 2017;4:30.


