Recent translational research into targeted therapy for liposarcoma
Introduction
Liposarcomas (LPS) are malignant tumors originating from mesenchymal cells (1-5). Although LPS can occur in almost anywhere in the body, over half develop in the thigh, and up to one-third involve the abdominal cavity (1-5). According to the WHO Classification of Soft Tissue and of Bone published in 2013, LPS can be divided into four types: well-differentiated liposarcoma (WDLPS), dedifferentiated liposarcoma (DDLSP), myxoid/round cell liposarcoma (MLPS), and pleomorphic liposarcoma (PLPS) (2-8).
The main feature of WDLPS is the excessive proliferation of adipocytes, while DDLPS includes both, a fusiform-cell-rich dedifferentiated portion and an adipocyte-rich well differentiated portion (1,3,5). WDLPS and DDLPS have both shown amplification of 12q13-15, which includes the MDM2 gene, but they demostrate different pathological features (9-13). In addition to surgical treatment, the most common types, WDLPS and DDLPS, show obvious resistance to conventional radiotherapy (RT) and cytotoxic chemotherapy (CT) (1,5). MLPS is another common subtype of LPS, with a 5-year overall survival rate of 90%, compared with 50% in patient with round cell LPS (14-17). Most MLPSs show the translocations t(12;22)(q13;q12) and t(12;16)(q13;p11.2), which lead to fusion of EWS-CHOP and FUS-CHOP (14,18-20) respectively.
There different histopathological and genetic features mean that LPS variants exhibit different aggressive potentials, reflecting their morphologic diversity. DDLPS, high-grade MLPS, and PLPS have a high propensity to metastasize, while ALT/WDLPS does not metastasize without dedifferentiation, and MLPS exhibits indolent clinical behavior and a lower metastatic potential (Table 1) (3,21).
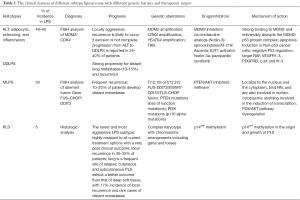
Full table
Treatment options of LPS patients involve surgery, CT, and RT. The goal of surgery, as the standard treatment for localized tumors, is to achieve complete tumor resection with negative margins. RT and CT, which can be administered pre- and/or postoperatively as a part of multimodal strategy for the management of localized tumors and they have shown controversial results (22). The standard treatment of metastatic disease is cytotoxic chemotherapy but it shows limited success. LPS sensitivity to CT seems to be correlated with the histologic subtype, and MLPS has a higher sensitivity to cytotoxic CT than other LPS subtypes (3). Histology and the primary site are the independent prognostic factors associated with survival in LPS patients (23). Novel and more effective systemic therapies are needed to meet the needs of LPS patients. Targeted therapy aims to exploit specific biologic features of the tumor in order to eradicate it. However, its rarity and the diverse molecular and genetic characteristics of each subtype are hindering the development of new targeted therapies (3,5,11,15,21). However, there have been several translational studies and trials in different subtypes of LPS (11,16,20,24-27).
12q13-15 amplicon and genetic amplification
Gene amplification in WD/DDLPS patients with chromosome 12q13-15 amplification may be a key event in the pathogenesis of LPS (28). Such amplified genes can be investigated by molecular biological methods and thus have the potential to act as a biomarker and target (29-31).
The MDM2 (also known as HDM2) gene is located at chromosome 12q15 and is amplified in most WD/DDLPS (Table 1) (32-34). Amplification of MDM2 inhibits the activity of p53, which leads to its loss of function as a tumor suppressor (35-37). Similarly, the cyclin dependent kinase-4 gene (CDK4) is also amplified in most WDLPS and DDLPS cases (Table 1) (29,32,38). At the molecular level, CDK4 inactivates retinoblastoma (Rb) protein and promotes cell-cycle transition from G1 phase to S phase (39). Similar to MDM2 and CDK4, the YEATS domain containing 4 gene (YEATS4) is also located on 12q13-q15 (Table 1). As a transcription factor participating in p53 regulation, YEATS4 has also shown promising potential in target therapy (17,29,32).
MDM2 inhibitors
Nutlins is the first potent and specific MDM2 inhibitor (40). It replaces p53 from MDM2 with an inhibitory concentration 50 of 100–300 nm. Nutlin-3a has been reported to influence the Rb pathway through activation of the transcription factor E2F1, cause apoptosis in p53-null tumor cells (41). Nutlin-3 thus demonstrates exciting prospects as a therapeutic target. Several other MDM2 inhibitors, such as AT-219 and Ascenta are also currently being developed (42).
Receptor tyrosine kinase inhibitors
A recent study showed that several receptors, including MET, IGFR, AXL, and EGFR were overexpressed in WD/DDLPS (Table 1). All these receptors may act as targets, and have already available small-molecule inhibitors (43). For instance, the oral VEGFR2 tyrosine kinase inhibitor apatinib had showed significant effect in advanced round cell LPS (44). The PDGFR beta-mediated pathway also plays a role in the progression of canine LPS, and may thus represent a promising target for adjuvant cancer therapies (45). Aurora kinases have recently been shown to be deregulated in human tumors, making them an attractive target for cancer therapy (46). Aurora kinase A, was also overexpressed in LPS and MLN8237 has proven to a selective and potent inhibitor of Aurora A, in a dose-dependent manner, suggesting that doses effectively and specifically targeted at Aurora A may be effective in tumor growth suppression (46).
FUS-DDIT3/EWSR1-DDIT3 fusion
The characteristics of MLPSs include frequent local recurrence and metastasis (16,47-49). MLPS tumors are also characterized by specific translocations of t(12;16) or t(12;22), resulting in fusion of FUS-DDIT3 or EWSR1-DDIT3, respectively (Table 1) (20,49,50). Three EWSR1/DDIT3 and nine FUS/DDIT3 fusion transcripts have been detected to date (1,14,17,19,20,49,50). Regarding the biological role of these fusions in LPS, Aman et al. demonstrated that FUS-DDIT3 protein expression was inversely correlated with the expression of cell proliferation-associated molecules (20). Suggesting that FUS-DDIT3 is the regulatory site involved in the development of MLPSs at the transcription and expression levels, these fusion oncogenes might be potentially powerful therapeutic targets, and detailed investigations are needed to develop them as novel treatment methods for LPS. Another specific TLS-CHOP fusion, caused by the translocation of t(12;16), presents in almost all the myxoid LPS (14). The TLS-CHOP fusion protein has three common types: type I (also known as type 7-2), type II (type 5-2), and type III (type 8-2) and p53 status has been reported to show an association with type II fusion in MLPS (14).
PI3K/Akt signaling pathway
PI3K-Akt pathway is a signal transduction pathway, which can promote the growth and survival of extracellular signals. This signaling pathway is highly regulated through a variety of mechanisms, and often interacts with other signaling pathways (24). Disruption of the PI3K-Akt pathway regulation can result in an increased signaling activity, which is linked to a range of diseases, such as type II diabetes and cancer (24).
In MLPSs, p110α catalytic subunit mutations of PI3K gene have been frequently detected and are associated with a poor prognosis (51). Other frequently mutated genes include PIK3CA (18% of MLPSs), TP53 (17% of PLPS), and NF1 (8% of PLPS) (51). PIK3CA mutations are also associated with a poor clinical prognosis and Akt activation in MLPSs (51). These results indicate that Akt pathway plays a potential role in MLPS, supporting the need for further studies of this histologic subtype, including the effects of PI3K inhibitors (Table 1) (24,51).
Demicco et al. also studied the PI3K-Akt pathway in 44 cases of round cell and myxoid LPS (26). Compared with purely mucinous tumors, tumors with round cell alterations, frequently have higher levels of IGF1R or PIK3CA activation. Moreover, PI3K-Akt pathway activation in round cell LPS is verified by the increase in p4EBP1 than that of myxoid LPS, because the p4EBP1 increase is closely related to activating events, such as PTEN loss, IGF1R expression, or mutation of PIK3CA. In conclusion, these data support an important role for the PI3K-Akt pathway in MLPSs (Table 1) (19,26).
CCAAT/enhancer binding protein (C/EBP-α)
The C/EBP-α is a transcription factor involved in blood cell differentiation. C/EBP-α mutation can reduce CCATT/enhancer binding protein alpha activity, leading to transition of myeloid antecedents. C/EBP-α can interact with CDK4 and CDK2 (25). In normal adipogenesis, C/EBP-α and its partner PPAR-gamma can promote each other’s expression and maintain high levels of mRNAs and differentiation (25). C/EBP-α and PPAR-γ are reported to be down-regulated in DD/WDLPS. It has also been reported that, grown in differentiating conditions, the DD cell lines lacked the induction of C/EBP-α expression, despite partial induction of PPAR-γ (25). Furthermore, PPAR-γ levels increased appropriately with the increase of C/EBP-α in the medium without PPAR-γ ligand (25). These results suggested that restoring or increasing C/EBP-α might be a promising therapeutic approach for DDLPS (25).
Calreticulin (CRT)
CRT is also known as calregulin, CaBP3, CRP55, ERp60 and calsequestrin-like protein, and is encoded by the CALR gene. It is expressed in many cancer cells and plays an important role in promoting macrophages to engulf hazardous cancerous cells (52). Most cells are undamaged because of the presence of another molecular signal that blocks CD47-CRT. Hence, blocking CD47 with antibodies may have a positive effect on the treatment of cancer. Anti-CD47 did not affect the function of normal cells while removing cancer cell in mouse models of non-Hodgkin’s lymphoma and myeloid leukemias.
A recent study showed that several genes located at 19p13.1-13.2 were highly expressed in DDLPS, including genes encoding, CRT, which can inhibit the differentiation of adipocytes. The expression of CRT was detected in 45 patients with LPS, including 15 patients with DDLPS. CRT knockdown by siRNA resulted in adipogenesis and reduced cell proliferation in DD cells (52). CRT and CD47 might thus be effective therapeutic utilities in LPS, especially DDLPS (Table 1).
Minor-groove DNA binders
Trabectedin (Ecteinascidin-743, ET743) is an alkylating agent isolated from Ecteinascidia turbinate, which affects cancer cells by damaging DNA (53). Gronchi et al. reported on a multi-center phase II trial of neoadjuvant trabectedin in MLPS patients, initiated by the National Cancer Institute (54). Three of 23 patients showed a pathological complete responseindicating that a 24-h intravenous infusion of 1.5 mg/m2 trabectedin every 3 weeks might be a good treatment for MLPS (54).
PNU-166196 (brostallicin) can also bind to the DNA minor groove and regulate the transcription of the FUS-DDIT3 gene (55). A recent phase II study of brostallicin in advanced soft tissue sarcomas performed by the EORTC suggested that brostallicin resulted in rare tumor response (56). More investigations are therefore needed to explore the role of DNA minor groove binders in LPS-targeted therapy.
Other potential targets
TOP2A, PTK7, and CHEK1 were overexpressed in 140 cases of LPS, including all subtypes and in LPS cell lines (27). In LPS cell lines resulted in increased cell proliferation and reduced invasiveness (27). Furthermore, point mutations in CTNNB1, CDH1, FBXW7and EPHA1 also represent potential oncogenic events in LPS cells (51). The C-MET amplification rate detected by ISH is 4.8% (3/62) in LPS. 7.1% (1/14) in myxoid LPS, 28.6% (2/7) in PLPS, and zero in other types of LPS (57). EGFR amplification ratio is 17.5% (14/80) in LPS. Nearly 3.6% (1/28) in DDLPS, 41.7% (5/12) in WDLPS, 62.5% (5/8) in PLPS and 17.6% (3/17) in other/unknown types of LPS) (57). At the same time, increased expression of C-KIT, EGFR, PD-L1, and PD-1+TILs has been validated in these LPS by immunohistochemistry, indicating the potential for target therapy and immunotherapy (57,58). Further, investigation of these genetic aberrations might help in the development of more therapeutic methods for LPS patients.
Conclusions
Genetic aberrations, such as the 12q13-15 amplicon, genetic amplification of MDM2, CDK4, TOP2A, PTK7, and CHEK1, point mutations in CTNNB1, CDH1, FBXW7, and EPHA1, and the fusion of FUS-DDIT3/EWSR1-DDIT3 contribute to the pathogenesis of LPS and might also represent good therapeutic candidates. Tyrosine kinase inhibitors targeting MET, AXL, IGFR, EGFR, VEGFR2, PDGFR-β and Aurora kinase signaling may also be effective in certain types of LPS. Disruption of the PI3K/Akt signaling pathway and deregulation of C/EBP-α with its partner PPAR-γ, also represent promising therapeutic methods for LPS trials. Furthermore, targeting interaction between calreticulin and CD47 may also lead to useful novel cancer treatments. All these potential new targeted approaches and promising immunotherapies may provide useful supplements to existing treatments for LPS.
Acknowledgements
The authors thank Dr. Shafat Hassan for his revision.
Funding: This work was partly supported by the National Nature Science Foundation of China (81372872 to J Yang, 81402215 to X Du and 81320108022 to K Chen), funds from IRT_14R40 to K Chen and 16JCYBJC24100 to J Yang.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dodd LG. Update on liposarcoma: a review for cytopathologists. Diagn Cytopathol 2012;40:1122-31. [Crossref] [PubMed]
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology 2014;46:95-104. [Crossref] [PubMed]
- De Vita A, Mercatali L, Recine F, et al. Current classification, treatment options, and new perspectives in the management of adipocytic sarcomas. Onco Targets Ther 2016;9:6233-46. [Crossref] [PubMed]
- Doyle LA. Sarcoma classification: an update based on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer 2014;120:1763-74. [Crossref] [PubMed]
- Dei Tos AP. Liposarcomas: diagnostic pitfalls and new insights. Histopathology 2014;64:38-52. [Crossref] [PubMed]
- Jo VY, Doyle LA. Refinements in Sarcoma Classification in the Current 2013 World Health Organization Classification of Tumours of Soft Tissue and Bone. Surg Oncol Clin N Am 2016;25:621-43. [Crossref] [PubMed]
- Rosenberg AE. WHO Classification of Soft Tissue and Bone, fourth edition: summary and commentary. Curr Opin Oncol 2013;25:571-3.
- Coindre JM. New WHO classification of tumours of soft tissue and bone. Ann Pathol 2012;32:S115-6. [Crossref] [PubMed]
- Kammerer-Jacquet SF, Thierry S, Cabillic F, et al. Differential diagnosis of atypical lipomatous tumor/well-differentiated liposarcoma and dedifferentiated liposarcoma: utility of p16 in combination with MDM2 and CDK4 immunohistochemistry. Hum Pathol 2017;59:34-40. [Crossref] [PubMed]
- Clay MR, Martinez AP, Weiss SW, et al. MDM2 and CDK4 Immunohistochemistry: Should It Be Used in Problematic Differentiated Lipomatous Tumors?: A New Perspective. Am J Surg Pathol 2016;40:1647-52. [Crossref] [PubMed]
- Hanes R, Grad I, Lorenz S, et al. Preclinical evaluation of potential therapeutic targets in dedifferentiated liposarcoma. Oncotarget 2016;7:54583-95. [PubMed]
- Hallin M, Schneider N, Thway K. Well-Differentiated Liposarcoma With Hibernoma-Like Morphology. Int J Surg Pathol 2016;24:620-2. [Crossref] [PubMed]
- Liu SY, Joseph NM, Ravindranathan A, et al. Genomic profiling of malignant phyllodes tumors reveals aberrations in FGFR1 and PI-3 kinase/RAS signaling pathways and provides insights into intratumoral heterogeneity. Mod Pathol 2016;29:1012-27. [Crossref] [PubMed]
- Antonescu CR, Tschernyavsky SJ, Decuseara R, et al. Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases. Clin Cancer Res 2001;7:3977-87. [PubMed]
- Conyers R, Young S, Thomas DM. Liposarcoma: molecular genetics and therapeutics. Sarcoma 2011;2011:483154.
- Kerr LT, Donoghue JF, Wilding AL, et al. Axitinib Has Antiangiogenic and Antitumorigenic Activity in Myxoid Liposarcoma. Sarcoma 2016;2016:3484673.
- Nishio J, Iwasaki H, Nabeshima K, et al. Cytogenetics and molecular genetics of myxoid soft-tissue sarcomas. Genet Res Int 2011;2011:497148.
- Nishio J. Contributions of cytogenetics and molecular cytogenetics to the diagnosis of adipocytic tumors. J Biomed Biotechnol 2011;2011:524067.
- de Graaff MA, Yu JS, Beird HC, et al. Establishment and characterization of a new human myxoid liposarcoma cell line (DL-221) with the FUS-DDIT3 translocation. Lab Invest 2016;96:885-94. [Crossref] [PubMed]
- Aman P, Dolatabadi S, Svec D, et al. Regulatory mechanisms, expression levels and proliferation effects of the FUS-DDIT3 fusion oncogene in liposarcoma. J Pathol 2016;238:689-99. [Crossref] [PubMed]
- Ghadimi MP, Liu P, Peng T, et al. Pleomorphic liposarcoma: clinical observations and molecular variables. Cancer 2011;117:5359-69. [Crossref] [PubMed]
- Sleijfer S, Ouali M, van Glabbeke M, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur J Cancer 2010;46:72-83. [Crossref] [PubMed]
- Oh YJ, Yi SY, Kim KH, et al. Prognostic Model to Predict Survival Outcome for Curatively Resected Liposarcoma: A Multi-Institutional Experience. J Cancer 2016;7:1174-80. [Crossref] [PubMed]
- LoPiccolo J, Blumenthal GM, Bernstein WB, et al. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat 2008;11:32-50. [Crossref] [PubMed]
- Wu YV, Okada T, DeCarolis P, et al. Restoration of C/EBPalpha in dedifferentiated liposarcoma induces G2/M cell cycle arrest and apoptosis. Genes Chromosomes Cancer 2012;51:313-27. [Crossref] [PubMed]
- Demicco EG, Torres KE, Ghadimi MP, et al. Involvement of the PI3K/Akt pathway in myxoid/round cell liposarcoma. Mod Pathol 2012;25:212-21. [PubMed]
- Gobble RM, Qin LX, Brill ER, et al. Expression profiling of liposarcoma yields a multigene predictor of patient outcome and identifies genes that contribute to liposarcomagenesis. Cancer Res 2011;71:2697-705. [Crossref] [PubMed]
- Forus A, Bjerkehagen B, Sirvent N, et al. A well-differentiated liposarcoma with a new type of chromosome 12-derived markers. Cancer Genet Cytogenet 2001;131:13-8. [Crossref] [PubMed]
- Italiano A, Bianchini L, Keslair F, et al. HMGA2 is the partner of MDM2 in well-differentiated and dedifferentiated liposarcomas whereas CDK4 belongs to a distinct inconsistent amplicon. Int J Cancer 2008;122:2233-41. [Crossref] [PubMed]
- Wang X, Asmann YW, Erickson-Johnson MR, et al. High-resolution genomic mapping reveals consistent amplification of the fibroblast growth factor receptor substrate 2 gene in well-differentiated and dedifferentiated liposarcoma. Genes Chromosomes Cancer 2011;50:849-58. [Crossref] [PubMed]
- Pires de Camargo V, van de Rijn M, Maestro R, et al. Other targetable sarcomas. Semin Oncol 2009;36:358-71. [Crossref] [PubMed]
- Saâda-Bouzid E, Burel-Vandenbos F, Ranchère-Vince D, et al. Prognostic value of HMGA2, CDK4, and JUN amplification in well-differentiated and dedifferentiated liposarcomas. Mod Pathol 2015;28:1404-14. [Crossref] [PubMed]
- Kashima T, Halai D, Ye H, et al. Sensitivity of MDM2 amplification and unexpected multiple faint alphoid 12 (alpha 12 satellite sequences) signals in atypical lipomatous tumor. Mod Pathol 2012;25:1384-96. [Crossref] [PubMed]
- Mentzel T, Palmedo G, Kuhnen C. Well-differentiated spindle cell liposarcoma ('atypical spindle cell lipomatous tumor') does not belong to the spectrum of atypical lipomatous tumor but has a close relationship to spindle cell lipoma: clinicopathologic, immunohistochemical, and molecular analysis of six cases. Mod Pathol 2010;23:729-36. [Crossref] [PubMed]
- Zhang S, Zhou L, Hong B, et al. Small-Molecule NSC59984 Restores p53 Pathway Signaling and Antitumor Effects against Colorectal Cancer via p73 Activation and Degradation of Mutant p53. Cancer Res 2015;75:3842-52. [Crossref] [PubMed]
- Tovar C, Rosinski J, Filipovic Z, et al. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci U S A 2006;103:1888-93. [Crossref] [PubMed]
- Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004;303:844-8. [Crossref] [PubMed]
- Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta 2002;1602:73-87. [PubMed]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell 1995;81:323-30. [Crossref] [PubMed]
- Ambrosini G, Sambol EB, Carvajal D, et al. Mouse double minute antagonist Nutlin-3a enhances chemotherapy-induced apoptosis in cancer cells with mutant p53 by activating E2F1. Oncogene 2007;26:3473-81. [Crossref] [PubMed]
- Polager S, Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer 2009;9:738-48. [Crossref] [PubMed]
- Millard M, Pathania D, Grande F, et al. Small-molecule inhibitors of p53-MDM2 interaction: the 2006-2010 update. Curr Pharm Des 2011;17:536-59. [Crossref] [PubMed]
- Peng T, Zhang P, Liu J, et al. An experimental model for the study of well-differentiated and dedifferentiated liposarcoma; deregulation of targetable tyrosine kinase receptors. Lab Invest 2011;91:392-403. [Crossref] [PubMed]
- Dong M, Bi J, Liu X, et al. Significant partial response of metastatic intra-abdominal and pelvic round cell liposarcoma to a small-molecule VEGFR-2 tyrosine kinase inhibitor apatinib: A case report. Medicine (Baltimore) 2016;95:e4368. [Crossref] [PubMed]
- Avallone G, Pellegrino V, Roccabianca P, et al. Tyrosine Kinase Receptor Expression in Canine Liposarcoma. Vet Pathol 2017;54:212-7. [Crossref] [PubMed]
- Nair JS, Schwartz GK. MLN-8237: A dual inhibitor of aurora A and B in soft tissue sarcomas. Oncotarget 2016;7:12893-903. [PubMed]
- Shurell E, Vergara-Lluri ME, Li Y, et al. Comprehensive adipocytic and neurogenic tissue microarray analysis of NY-ESO-1 expression - a promising immunotherapy target in malignant peripheral nerve sheath tumor and liposarcoma. Oncotarget 2016;7:72860-7. [PubMed]
- Saponara M, Stacchiotti S, Gronchi A. The safety and efficacy of trabectedin for the treatment of liposarcoma or leiomyosarcoma. Expert Rev Anticancer Ther 2016;16:473-84. [Crossref] [PubMed]
- Safavi S, Jarnum S, Vannas C, et al. HSP90 inhibition blocks ERBB3 and RET phosphorylation in myxoid/round cell liposarcoma and causes massive cell death in vitro and in vivo. Oncotarget 2016;7:433-45. [PubMed]
- Rao UN, Cieply K, Sherer C, et al. Correlation of Classic and Molecular Cytogenetic Alterations in Soft-Tissue Sarcomas: Analysis of 46 Tumors With Emphasis on Adipocytic Tumors and Synovial Sarcoma. Appl Immunohistochem Mol Morphol 2017;25:168-77. [Crossref] [PubMed]
- Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet 2010;42:715-21. [Crossref] [PubMed]
- Hisaoka M, Matsuyama A, Nakamoto M. Aberrant calreticulin expression is involved in the dedifferentiation of dedifferentiated liposarcoma. Am J Pathol 2012;180:2076-83. [Crossref] [PubMed]
- Erba E, Bergamaschi D, Bassano L, et al. Ecteinascidin-743 (ET-743), a natural marine compound, with a unique mechanism of action. Eur J Cancer 2001;37:97-105. [Crossref] [PubMed]
- Gronchi A, Bui BN, Bonvalot S, et al. Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol 2012;23:771-6. [Crossref] [PubMed]
- Broggini M, Marchini S, Fontana E, et al. Brostallicin: a new concept in minor groove DNA binder development. Anticancer Drugs 2004;15:1-6. [Crossref] [PubMed]
- Leahy M, Ray-Coquard I, Verweij J, et al. Brostallicin, an agent with potential activity in metastatic soft tissue sarcoma: a phase II study from the European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Eur J Cancer 2007;43:308-15. [Crossref] [PubMed]
- Movva S, Wen W, Chen W, et al. Multi-platform profiling of over 2000 sarcomas: identification of biomarkers and novel therapeutic targets. Oncotarget 2015;6:12234-47. [Crossref] [PubMed]
- Available online: http://www.cancerresearch.org/cancer-immunotherapy/impacting-all-cancers/sarcoma
Cite this article as: Patel RB, Li T, Liao Z, Jaldeepbhai JA, Perera HA, Muthukuda SK, Dhirubhai DH, Singh V, Du X, Yang J. Recent translational research into targeted therapy for liposarcoma. Stem Cell Investig 2017;4:21.


