Inappropriate trafficking of damaged mitochondria in Parkinson’s disease
Mitochondria are essential for cellular function due to their involvement in ATP production by oxidative phosphorylation, lipid metabolism assembly, regulation of homeostasis and programmed cell death. It is widely recognized that mitochondrial dysfunction occurs in many neurodegenerative diseases.
In Parkinson’s disease (PD), mitochondrial dysregulation in the form of reduced complex I activity, increased mitochondria-derived reactive oxygen species (ROS) production, ROS mediated mitochondrial DNA damage, bioenergetic failure and perturbation of mitochondrial dynamics and mitophagy have long been implicated (1). In particular, the identification of genes linked to rare familial variants of PD revealed a common pathway involving mitochondrial dynamics, transport, turnover and mitophagy. This encourages research on mitochondrial aspects of disease etiopathogenesis.
Mitochondria are highly mobile and dynamic organelles and their movement along the microtubule can be adjusted according to the changes in cell activity and local energy command. Principally, neuronal cells depend on efficient trafficking of mitochondria to supply distant subcellular locations between the cell body and axon terminal. In the process of energy production, mitochondria become filled with toxic and cell damaging free radicals. Mitochondria get damaged as they work and their function becomes gradually less efficient, eventually to a point that the mitochondria are making more free radicals than energy, translating into more and more oxidative stress. Mitophagy is a highly efficient system for removing damaged mitochondria. Defects in this process are associated with neurodegeneration.
Mitochondrial GTPase Miro is an outer mitochondrial membrane (OMM) protein that facilitates mitochondrial transport by attaching the mitochondria to the microtubule via its binding partners Milton and kinesin heavy chain (KHC). Prior to clearance of damaged mitochondria by mitophagy, Miro is removed from the mitochondrial membrane, resulting in loss of mitochondrial motility and mitochondrial arrest (2).
In a recent paper by Hsieh and colleagues, functional impairment in Miro degradation and mitophagy is reported to be a shared feature in familial and sporadic PD (3). The authors first uncovered impairment in Miro degradation and subsequent damaged mitochondrial clearance in skin fibroblasts derived from sporadic and also familial PD patients with confirmed mutations in Leuchine-rich repeat kinase 2 (LRRK2), PTEN-induced putative kinase 1 (PINK1) and Parkin. They then differentiated induced pluripotent stem cells (iPSC) reprogrammed from three PD patients harboring LRRK2G2019S mutations, the most common known genetic contributor to PD, to tyrosine hydroxylase (TH)-positive-dopaminergic neurons that represent an ideal model for validation. LRRK2G2019S delays Miro removal and mitochondrial arrest and subsequent clearance in response to mitochondrial depolarization in iPSC-derived axons. Exploiting the previous relation of PINK1 and Parkin to Miro, they found wild type LRRK2 promotes Miro removal from OMM by forming a complex with Miro. By a loss of function, LRRK2G2019S disrupts interaction with Miro on damaged mitochondria and delays initiation of mitophagy. More importantly, partial reduction of Miro using RNA interference (RNAi) arrests damaged mitochondria, restores mitophagy and protects against mitochondrial stress in LRRK2G2019S iPSC-derived neurons. They further tested the approach of Miro knockdown in vivo using Drosophila overexpressing LRRK2G2019S. Locomotor defects and dopaminergic neurodegeneration in Drosophila expressing LRRK2G2019S were rescued by partial reduction of Miro. These findings highlight involvement of Miro proteins in mitochondria turnover which emerges as a key process affected in PD-associated neurodegeneration. The authors’ efforts provided more than just evidence of how dopaminergic neurons in substantia nigra die as they also found a new way to prevent it from happening. By lowering Miro expression, the threshold for mitochondria detachment from microtubule is lowered. This facilitates disassembly of damaged mitochondria and prevents neuronal cell death.
Parkin, the E3 ubiquitin ligase, and PINK1, the serine/threonine kinase are two other proteins known to be mutated in certain forms of early onset PD. Parkin and PINK1 cooperate in a biochemical mitochondrial quality control pathway regulating mitochondrial morphology, dynamics and clearance. As they were the first two proteins implicated in mitophagy, there have been diverse opinions with little consensus on their precise roles in this process. In the old model of PINK1/Parkin-mediated mitophagy, PINK1 acts upstream of Parkin and has a small initiator role of phosphorylating ubiquitin to activate the ubiquitin ligase Parkin, which builds ubiquitin chains on mitochondrial outer membrane proteins where they act to recruit autophagy receptors (4). It has been recently revealed that PINK1 has a new and more central role to activate mitophagy directly by generating phospho-ubiquitin on mitochondria to recruit autophagy receptors NDP52 and optineurin. Parkin serves to amplify the PINK1 generated phospho-ubiquitin signal to allow for robust and rapid mitophagy induction (5).
PD-patient-specific Parkin- and PINK1 iPSC-derived midbrain neurons recapitulate PD phenotypes including abnormal mitochondrial morphology and impaired mitochondrial homeostasis. Control mitochondria showed a characteristically long, cylindrical profile with well-organized cristae, and the electron density of the matrix was relatively low. By contrast, mitochondria in PD-patient-specific Parkin- and PINK1 iPSC-derived midbrain neurons showed signs of swelling with irregular and dilated cristae. The relative perikaryal volume of the abnormal mitochondria was significantly increased, and that of the normal mitochondria was decreased compared with control neurons (6,7). Mitochondrial alteration was accompanied by reduction in the percentage of dopaminergic neurons in PD patient-derived neurons carrying various mutations in Parkin compared with age-matched control subject (8).
Interestingly, a previous report from the authors’ group reported PINK1 and Parkin regulate mitochondrial trafficking and quarantine damaged mitochondria by severing their connection to the microtubule network. Miro is one of the substrates of PINK1. PINK1 and Parkin associate with motor/adaptor Miro on depolarized mitochondria. Phosphorylation of Miro by PINK1 activates proteasomal degradation of Miro through a Parkin-dependent mechanism. Destruction of Miro unhooks damaged mitochondria from the microtubule network, preventing their transport throughout the cell (9). This finding suggests PINK1/Parkin pathway may quarantine damaged mitochondria prior to their clearance. In agreement with the abovementioned findings, alteration in Miro1 turnover on damaged mitochondria has been reported in PD patient-derived fibroblasts containing a pathogenic mutation in Parkin. They further showed Miro ubiquitination is dependent on the Ser-65 residue within Parkin that is phosphorylated by PINK1, indicating that disruption of this regulation may be implicated in PD pathogenesis (10).
In Drosophila model, PINK1 mutant flies exhibit abnormal wing postures, reduced flight ability and thoracic ATP level, degeneration of indirect flight muscle and dopaminergic neurons, and male sterility, which are caused by the accumulation of dysfunctional mitochondria. Downregulation of Miro or other components of the mitochondrial transport machinery rescued PINK1 mutant phenotypes in the muscle and dopaminergic neurons, whereas Miro overexpression alone caused dopaminergic neuron loss. Miro protein level was increased in PINK1 mutant but decreased in PINK1 or Parkin overexpression models (11). PINK1 was also found to inhibit synaptic growth and protect dopaminergic neurons by phosphorylating Miro in Drosophila. Expression of phospho-resistant Miro in a Miro null mutant background phenocopied a subset of phenotypes of PINK1 null flies. Specifically, phospho-resistant Miro increased mitochondrial movement and synaptic growth at larval neuromuscular junctions, and decreased the number of dopaminergic neurons in adult brains (12).
These newly identified relationship of LRRK2 and PINK1/Parkin with Miro in mitochondrial transport and mitophagy contribute to our understanding of the complex interplays in mitochondrial quality control that are critically involved in PD pathogenesis (Figure 1).
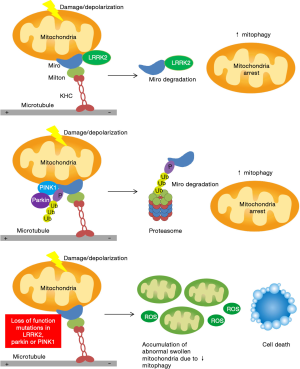
In healthy mitochondria, Miro and its binding partners connect mitochondria to microtubules, facilitating trafficking of the organelles to various cellular locations. Upon mitochondrial damage, PINK1 levels are stabilized on the outer membrane, resulting in the recruitment of Parkin and modification of Miro. Through phosphorylation, ubiquitination and proteasomal pathways, Miro is degraded. The stationary damaged mitochondrion is segregated and can then undergo mitophagy. PINK1/Parkin pathway is not affected by LRRK2G2019S while LRRK2 binding to Miro and recruitment to damaged mitochondria are not affected by PINK1/Parkin mutations, implying that LRRK2 and the PINK1/Parkin pathway function in parallel and converge on Miro. Overexpression of Parkin in LRRK2G2019S fibroblasts failed to rescue the impaired Miro degradation phenotype, indicating the each pathway may not compensate for defect in the other (3).
In conclusion, existing drugs for PD therapies may significantly improve quality of life but they do not slow or stop progressive dopaminergic neuronal death, the hallmark feature of the disease. Advances in iPSC technology enable us to recapitulate the pathogenic changes including mitochondria abnormalities in the brain of PD patients. Collectively, the evidences add up to a case of inappropriate trafficking of damaged mitochondria as a significant role in PD pathogenesis. These novel findings bring us a step closer to understand the cause of PD and mechanistic insights into the onset of disease and thereby facilitate the identification of potential modified therapies for PD. In PD, progressive dopaminergic neurodegeneration and mitochondrial abnormalities are thought to begin long before motor symptoms appear. Preferably, treatment should begin in patients who are in early stages of disease development whereby the main cell type expressing the defective gene still intact and can be targeted. However, the variability of symptom severity, type and rate of progression among patients makes diagnosis difficult especially in the early stages. Therefore, there is a need for reliable and validated biomarkers to identify earlier stage patients. With the new finding of Miro being resistant to degradation in PD patients with a wide range of backgrounds, measurement of Miro levels in skin fibroblasts from people at risk of PD as biomarker may be helpful in getting precise and early diagnosis. Furthermore, targeting Miro by RNAi induced gene knockdown may give therapeutic benefit and thereby open new perspective and perk up development of novel strategies in the field of PD gene therapy treatment. As patient-derived skin fibroblasts and iPSC cells are easily accessible, they also provide a great source to screen for therapeutic interventions. Subsequent feasible approach may involve screening for small-molecule compounds that downregulate Miro level using patient-derived cells for efficient PD treatment.
Acknowledgements
Funding: This research is supported by the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) and The Practical Research Project for Rare/Intractable Diseases (15ek0109029h0002) from Japan Agency for Medical Research and development, AMED, Grant-in-Aid for Scientific Research on Innovative Areas (Brain Protein Aging and Dementia Control) (15H01558) from MEXT, Grants-in-Aid from the Research Committee of CNS Degenerative Diseases, the Ministry of Health, Labour and Welfare of Japan and JSPS KAKENHI Grant Number 26293210.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Perier C, Vila M. Mitochondrial biology and Parkinson's disease. Cold Spring Harb Perspect Med 2012;2:a009332. [Crossref] [PubMed]
- Ashrafi G, Schlehe JS, LaVoie MJ, et al. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol 2014;206:655-70. [Crossref] [PubMed]
- Hsieh CH, Shaltouki A, Gonzalez AE, et al. Functional Impairment in Miro Degradation and Mitophagy Is a Shared Feature in Familial and Sporadic Parkinson's Disease. Cell Stem Cell 2016;19:709-24. [Crossref] [PubMed]
- Vives-Bauza C, Zhou C, Huang Y, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A 2010;107:378-83. [Crossref] [PubMed]
- Lazarou M, Sliter DA, Kane LA, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 2015;524:309-14. [Crossref] [PubMed]
- Chung SY, Kishinevsky S, Mazzulli JR, et al. Parkin and PINK1 Patient iPSC-Derived Midbrain Dopamine Neurons Exhibit Mitochondrial Dysfunction and α-Synuclein Accumulation. Stem Cell Reports 2016;7:664-77. [Crossref] [PubMed]
- Imaizumi Y, Okada Y, Akamatsu W, et al. Mitochondrial dysfunction associated with increased oxidative stress and α-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol Brain 2012;5:35. [Crossref] [PubMed]
- Shaltouki A, Sivapatham R, Pei Y, et al. Mitochondrial alterations by PARKIN in dopaminergic neurons using PARK2 patient-specific and PARK2 knockout isogenic iPSC lines. Stem Cell Reports 2015;4:847-59. [Crossref] [PubMed]
- Wang X, Winter D, Ashrafi G, et al. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 2011;147:893-906. [Crossref] [PubMed]
- Birsa N, Norkett R, Wauer T, et al. Lysine 27 ubiquitination of the mitochondrial transport protein Miro is dependent on serine 65 of the Parkin ubiquitin ligase. J Biol Chem 2014;289:14569-82. [Crossref] [PubMed]
- Liu S, Sawada T, Lee S, et al. Parkinson's disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet 2012;8:e1002537. [Crossref] [PubMed]
- Tsai PI, Course MM, Lovas JR, et al. PINK1-mediated phosphorylation of Miro inhibits synaptic growth and protects dopaminergic neurons in Drosophila. Sci Rep 2014;4:6962. [Crossref] [PubMed]
Cite this article as: Choong CJ, Mochizuki H. Inappropriate trafficking of damaged mitochondria in Parkinson’s disease. Stem Cell Investig 2017;4:17.


