Producing tissue specific stem cells for regeneration: how YAP/TAZ may prove useful
In humans, many organs fail to repair through endogenous mechanisms following injury. However, we know from developmental studies, and from research in other model organisms, that tissue regeneration is possible for many organs. For these reasons, regenerative medicine is constantly on the hunt for molecules that can unlock the natural ability of tissues to repair themselves. The homologous co-transcriptional regulators YAP and TAZ may be key molecules capable of resetting mature differentiated cells into a more regenerative state.
YAP and TAZ, endpoints of canonical Hippo signaling, are transcription modulators that bind and regulate the activity of DNA-binding transcriptions factors (1). YAP and TAZ usually function interchangeably, and most commonly bind members of the TEAD family of transcription factors. They are key in regulating the switch between a proliferative and non-proliferative cell fate, but also regulate other cellular decisions and processes such as cell type determination, cytoskeletal dynamics, and cell-cell or cell-matrix interactions (2). While many progenitors express either YAP and/or TAZ, most mature cells lose expression of these proteins (3-5). Generally, dephosphorylated YAP/TAZ is active and leads to tissue growth. Additionally, YAP/TAZ have been shown to control the formation of cancer stem cells (6,7), and activation of YAP/TAZ can affect the severity of several cancer types, such as liver cancers (8), and uveal melanoma (9,10). Although YAP/TAZ have been clearly demonstrated to be required for regeneration in natural regenerating tissues such as the lung (11), heart (12), and liver (13), the potential of ectopic YAP/TAZ to induce regeneration in tissues that do not normally regenerate has not been investigated.
The discovery that expression of a key set of transcription factors can turn nearly any mature differentiated cell into an induced pluripotent stem cell (iPSC) (14) has revolutionized regenerative medicine, and provided a powerful tool for modeling human disease. However, creating iPSCs using the well-known Yamanaka factors without careful directed differentiation has several potential drawbacks. Critically, uncontrolled differentiation of iPSCS and subsequent implantation can lead to the formation of teratomas as their cells fates are unrestricted (15). Furthermore, many of the best directed differentiation protocols frequently produce cultures that contain a low purity of the desired cell type. Challenges with directed differentiation may result from the inability of Yamanaka factors to reinstate all of the cellular and epigenetic hallmarks characteristics of truly “ground state” pluripotent stem cells (16). An alternative strategy has been to directly reprogram one cell type into another. This has been shown possible for several neuronal subtypes (17), and is a potential method to overcome the difficulty in precise differentiation of iPSCs. However, direct reprograming has its own draw backs in that it requires a very precise set of transcription factors, and only enables replacement of a lost cell type, rather than regeneration of an entire tissue. Formation of tissue-specific stem cells, which are more restricted than iPSCs, but still able to produce all cells needed to regenerate a specific tissue, may prove to be a key strategy to overcome the draw backs of both of these methods and move regenerative medicine forward.
Panciera et al. investigated the potential of YAP/TAZ to form tissue specific stem cells (18). They show that activation of YAP/TAZ is one potential mechanism to induce the formation of tissue specific stem cells with promising regenerative capacity to reform tissues that do not naturally repair. Figure 1 illustrates several of the key findings of this manuscript in the context of the cellular reprograming field. The authors first investigate the ability of YAP/TAZ to transform luminal cells into tissue specific mammary stem cells. To do this, they used fluorescence-activated cell sorting to isolate luminal cells from mammary gland of mice, infected the isolated cells with lentiviral vectors expressing activated forms of YAP or TAZ under the control of a doxycycline inducible promoter. Mature luminal cells grew as a monolayer in culture, but with doxycycline treatment and activation of exogenous YAP, these cells formed spherical colonies that appeared identical to natural mammary stem cell cultures. In particular, these organoids could be passaged, demonstrating the presence of induced stem cells. Importantly, tissue specific stem cells were maintained even after removal of doxycycline, indicating that ectopic YAP/TAZ activation is only required for the initial induction of the stem cell state. In an exciting finding, these YAP/TAZ-induced mammary stem cells were able to form all the components of mammary tissue in both an organoid culture system, and when injected into adult ‘cleared’ mammary fat pads, where development of the endogenous epithelial ductal tree was prevented. When injected into the cleared mammary fat pads of these mice, the cells formed functional mammary tissue.
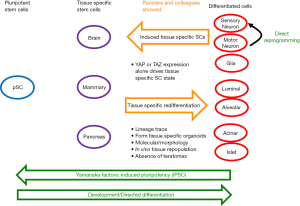
To investigate the general ability of YAP/TAZ to induce tissue-specific stem cells, the authors also studied the effects on neurons and pancreatic cells. Both the central nervous system and pancreas have limited regenerative ability naturally. In either neurons or pancreatic cells, induced expression of active YAP/TAZ resulted in formation of tissue-specific stem cells. These cells could be passaged, and retained stem cell characteristics. As with mammary epithelia, only transient expression of YAP/TAZ was required. Transcriptomic analysis indicated that exogenous expression of YAP/TAZ activated longer lasting endogenous expression of these factors. The importance of induced endogenous expression of YAP/TAZ was demonstrated by RNAi depletion studies. The inability for induced tissue specific stem cells to maintain their regenerative potential when YAP/TAZ were depleted further indicates the key role of these proteins in maintenance of the stem cell state. One key difference in the neuronal experiment is that induced neurons were cultured from prenatal mice since postnatal neurons are not able to be cultured. However, the neurons studied were post-mitotic, so although these findings are indicative that YAP-induced reprograming occurs in neurons, further work is required to investigate how this works on adult neurons. The YAP-induced pancreatic stems cells were able to form ductal organoids comprised of multiple cell types. Additionally, the YAP-induced neuronal stems were able to generate neuronal and glial cells in both tissue culture and implantation experiments. Importantly, in all of the implantation experiments performed the authors saw no signs of tumor formation.
From these results, it is reasonable to suspect that YAP/TAZ induction of stem cell fate produces a more restricted pluripotent stem cell. This could offer several advantages to other methods, such as those that use Yamanaka factors, in particular, reduced risk of tumor formation. However, the restricted fate itself could limit their utility in regenerative medicine, as patients are unlikely to donate their brain tissue.
Panciera et al. clearly show the ability of YAP/TAZ-induced iPSCs (Y-iSCs) to generate the variety of cell types required to form mammary tissue in a post-embryonic state. What is less certain is whether Yap-induced tissue specific SCs can form functional neuronal and pancreatic cells. When Y-iSCs are transplanted into the brains of mouse pups the majority appear to become astrocytes. Although some cells stain for generic neuronal markers, it is unclear if they become appropriate neuronal sub-types for their location within the brain, or can make functional synaptic connections. This does not detract from the importance of their work, but future research is needed to determine if Y-iSCs can be differentiated into specific neuronal types, or if they naturally take on the required identity of the surrounding environment.
The mechanism by which YAP/TAZ are able to induce stem cell formation also requires further study. As with other ground-breaking studies, this one too raises many questions. Does YAP/TAZ activation regulate any of the Yamanaka factors, or do these mechanisms overlap in some other manner? How does induced YAP/TAZ activation lead to activation of endogenous YAP/TAZ? Does YAP/TAZ recruit chromatin remodeling or DNA modifying factors that allow cells to revert to a stem state in which more pluripotent associated genes are expressed. What are the key genes that are regulated by YAP/TAZ to induce the stem cell state? Why do only a sub-set of cells (18% for breast epithelia) respond to YAP/TAZ induction by reinitiating progenitor programs? Related, why are some tissues able to activate YAP/TAZ to regenerate the tissue naturally, but the cell types studied here are not, even though they appear equally capable to do so?
To determine if YAP/TAZ induction of regeneration is likely to prove useful in regenerative medicine the authors acknowledge it must first be determined if this mechanism works in human tissue. Furthermore, it will be important to see if YAP/TAZ stem cell induction can work in vivo since the induction was only tested in vitro in this manuscript. In particular, for age-related neurodegenerative diseases, it will be critical to test the ability of YAP induction to transform neurons from adults into stem cells, as there is great need to regenerate adult tissue. More broadly for age-related disease in general, there is urgency to understand how universal the ability of YAP is to induce stem cells formation.
Overall, this recent work, combined with previous experiments, shows that regulation of YAP/TAZ could be a key regulator of regenerative capacity. Furthermore, the addition of YAP/TAZ-induced tissue-specific stem cells to iPSCs production and direct reprogramming expands the regenerative biology toolset. It will be exciting to chart the impact of these findings on regenerative medicine and development of new scientific tools.
Acknowledgements
The authors thank members of the Link and Besharse Labs for helpful discussions.
Funding: This work was supported by grants R01EY014167 (BA Link), R01EY016060 (BA Link) and F32EY024184 (SJ Henle).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 2014;141:1614-26. [Crossref] [PubMed]
- Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 2014;94:1287-312. [Crossref] [PubMed]
- McNeill H, Reginensi A. Lats1/2 Regulate Yap/Taz to Control Nephron Progenitor Epithelialization and Inhibit Myofibroblast Formation. J Am Soc Nephrol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Elbediwy A, Vincent-Mistiaen ZI, Spencer-Dene B, et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development 2016;143:1674-87. [Crossref] [PubMed]
- Kim T, Yang SJ, Hwang D, et al. A basal-like breast cancer-specific role for SRF-IL6 in YAP-induced cancer stemness. Nat Commun 2015;6:10186. [Crossref] [PubMed]
- Song S, Ajani JA, Honjo S, et al. Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties. Cancer Res 2014;74:4170-82. [Crossref] [PubMed]
- Cordenonsi M, Zanconato F, Azzolin L, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 2011;147:759-72. [Crossref] [PubMed]
- Zhou D, Conrad C, Xia F, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 2009;16:425-38. [Crossref] [PubMed]
- Yu FX, Luo J, Mo JS, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell 2014;25:822-30. [Crossref] [PubMed]
- Feng X, Degese MS, Iglesias-Bartolome R, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell 2014;25:831-45. [Crossref] [PubMed]
- Liu Z, Wu H, Jiang K, et al. MAPK-Mediated YAP Activation Controls Mechanical-Tension-Induced Pulmonary Alveolar Regeneration. Cell Rep 2016;16:1810-9. [Crossref] [PubMed]
- Xin M, Kim Y, Sutherland LB, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A 2013;110:13839-44. [Crossref] [PubMed]
- Hong L, Cai Y, Jiang M, et al. The Hippo signaling pathway in liver regeneration and tumorigenesis. Acta Biochim Biophys Sin (Shanghai) 2015;47:46-52. [Crossref] [PubMed]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76. [Crossref] [PubMed]
- Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, et al. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells 2010;28:1568-70. [Crossref] [PubMed]
- Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010;467:285-90. [Crossref] [PubMed]
- Masserdotti G, Gascón S, Götz M. Direct neuronal reprogramming: learning from and for development. Development 2016;143:2494-510. [Crossref] [PubMed]
- Panciera T, Azzolin L, Fujimura A, et al. Induction of Expandable Tissue-Specific Stem/Progenitor Cells through Transient Expression of YAP/TAZ. Cell Stem Cell 2016;19:725-737. [Crossref] [PubMed]
Cite this article as: Henle SJ, Link BA. Producing tissue specific stem cells for regeneration: how YAP/TAZ may prove useful. Stem Cell Investig 2017;4:16.


