A translational approach to clinical practice via stress-responsive glucocorticoid receptor signaling
Introduction
A research approach that describes reliable neurobiological findings based on psychopathological syndromes will be more solid contrasted to a nonetiologic system of classification. Integrative approaches to understanding complex health issues can transcend disciplinary and knowledge boundaries and provide opportunities to view phenomena from diverse perspectives. A future diagnostic criteria system in which etiology and pathophysiology are essential in diagnostic decision making would bring psychiatry closer to other specialties of medicine. The relationship between stress and illness is a strong example of a field of study that can be more fully understood from an integrative perspective (1).
This approach says very clearly and without a doubt that the causes, development, and outcomes of disorders are determined by the relations of psychological, social, and cultural factors with biochemistry and physiology. Biochemistry and physiology are not disconnected and different from the rest of our experiences and life events (2).
It is now broadly accepted that psychological stress may change the internal homeostatic state of an individual. During acute stress, adaptive physiological responses occur, which include increased adrenocortical secretion of hormones, primarily cortisol. This action of the brain is the body’s first line of defense against illness, against aging, and for health and welfare (3).
As we know, stress is vital for the adaptation of life on Earth. Although usually associated with disease, the physiological and behavioral responses to stressors are critical mechanisms of resilience for healthy organisms (4).
A stressor (or stressful event) can be defined as a physical (or psychological) stimulus that disturbs or threatens to disturb homeostasis. As stressors are omnipresent, stress is vital for survival. Sometimes, however, depending on the duration, intensity, and subject vulnerability, stress can induce physical or psychological dysfunction. Approximately 60% of cases of depressive episodes are preceded by stressors, especially psychosocial ones (5).
Genes, early life stress (ELS), adult experiences, life style, and stressful life experiences all add to the way the body adapts to a changing environment; and all these factors help to determine the cost to the body or the “allostatic load”. Emergent data in the field of psychoneuroimmunology contributes to the understanding of the mechanisms by which traumatic events affect health. The interaction between behavior, neurobiology, and endocrine system that may cause immunosuppression is the most interesting discovery in current medicine, and its implications are important for the prevention and treatment of somatic diseases (6).
Faced with a stressful situation the organism will elicit an immediate response via activation of the autonomous nervous system (ANS) and a delayed response via hypothalamic–pituitary–adrenal (HPA) axis-mediated release of glucocorticoids (GC) (7). These hormones will in turn have easy access to the brain and, once there, will bind to mineralocorticoid receptors (MR) and glucocorticoid receptors (GR) to exert both rapid, non-genomic and slow, genomic actions on physiology and behavior (8). GRs are highly expressed in most brain regions, whereas MRs are predominantly expressed in limbic regions such as the hippocampus and central amygdala. GR activation has been linked to adaptive processes such as memory consolidation, whereas MR-mediated effects have been associated with the appraisal and responsiveness to stressful experiences and with the negative feedback control of the HPA axis (9,10).
One thing that is important to realize is that different kinds of stress will elicit that response (although by different inputs) whether they are hypoxic, metabolic, inflammatory, hematologic or even psychogenic or “imagined” stressful situations (7).
Thus, it is clear that the HPA axis constitutes one of the major endocrine systems that maintain homeostasis when the organism is challenged or stressed and activation of the HPA axis is perhaps the most important endocrine component of the stress response (11).
Epigenetics and ELS
ELS is an important, although non-specific, risk factor for major psychiatric and medical disorders, and that includes intrauterine stress as well (12,13). Stress in pregnancy has been shown to have a programming effect in the offspring, and one of the most consistent findings is HPA axis alterations (14). Although GC plays a vital role in the development of the embryo, prolonged exposures may have deleterious neural effects, especially in brain areas that are rich in cortisol receptors (14). Consistent with the findings of Kwan et al. (15) prenatal SSRI exposure of mothers seems to alter HPA axis function and cortisol levels (16) as well as neonatal corticosteroid binding globulin (CBG) in the infants (17). CBG is an important mediator of cortisol function, since only the free cortisol appears to exert its physiological effects. In normal conditions more than 90% of circulating cortisol is bound to CBG (18). Mice exposed to SSRIs also show altered hormonal and behavioral response to stress, supposedly mediated by GC resistance (19).
Animal studies suggest that maternal immune activation increase the risk of behavioural deficits (20) and depression-like behaviour in the offspring (21). Since GCs are thought to modulate immune function and that these hormones can have important epigenetic effects (22), it is now believed that this alterations can be passed on even intergenerationally (12).
In an already classic animal study, Dias and Ressler demonstrated that rats submitted to an olfactory cue and subsequently shocked passed on their behavioural reaction to the same odour to their offspring that had not had contact with the smell nor the shocks, but nevertheless showed an increased startle response to the olfactory cue. Epigenetic mechanisms are thought to be involved and GC is one plausible effector candidate (23). Another animal study suggests that paternal exposure to stress can alter sperm microRNA content and have programming effects on the HPA axis of their offspring as well (24).
Humans are probably subject to the same mechanisms, as suggested by studies of the great Dutch famine of 1944 to 1945. These studies showed that prenatal maternal malnutrition was associated with a variety of physical conditions in offspring when adults (25), as well as an increased risk of schizophrenia spectrum disorder and depression (26). The offspring of fathers that were intrautero at that time (grandchildren of the malnourished) were found to have increased risk of obesity, suggesting a transgenerational effect (27).
These studies are mentioned only to illustrate the magnitude and complexity of the effects of ELS, and when that can happen, being in childhood, intrautero or, it seems, even before conception takes place. Clearly, more studies are needed to elucidate the precise mechanisms of these effects and their relevance in human development, physical and mental health.
Studies of childhood maltreatment and their persistent effects throughout the lifespan have been more studied and from now on we will refer to ELS as stress that occurs in early childhood.
In agreement with Bernstein et al. (28), childhood maltreatment may be subdivided into the following domains:
- Physical abuse: physical aggression by someone older, with the risk of or result of injury;
- Emotional abuse: verbal aggression that affects the welfare or morale of the child or any conduct that humiliates, embarrasses, or threatens the child;
- Sexual abuse: any type of sexual contact or conduct between a child and someone older;
- Emotional neglect: failure of caretakers to provide for basic emotional and psychological needs such as love, motivation, and support;
- Physical neglect: failure of caretakers to provide for basic physical needs such as feeding, a home, security, supervision, and health.
Childhood maltreatment significantly contributes to disease morbidity and mortality in adults (29,30) and it is essential to elucidate the mechanisms by which these early life events can elicit illnesses that become apparent decades after the presumed initial insult, and why some people can adapt and others will present with an increased risk for psychiatric disorders, especially depression. It seems that a complex interaction between genes and environment are responsible for these effects.
Polymorphisms in genes that code for 5-HT transporters have already been implicated in increased susceptibility for depression, when interacting with an environmental stressor, following the work of Caspi (31). More recent papers show that these effects may also be mediated by the HPA axis (32), specially through the adrenal gland (33).
ELS, HPA axis and corticoid receptors
Dysregulation of the HPA axis is one of the most consistent findings in patients with depression and ELS (34). Patients with ELS are more likely to show hyperactivity of the HPA axis and present symptoms that are usually resistant to standard antidepressants but instead benefit from adjuvant treatment with psychotherapy (35-37).
Evidence indicates that stress in the early phases of development can induce persistent changes in the ability of the HPA axis to respond to stress in adulthood, and that this mechanism can lead to a raised susceptibility to depression. These abnormalities appear to be related to changes in the ability of circulating GCs to exert negative feedback on the secretion of HPA hormones through binding to GR and MR (18).
In humans, while MRs are thought to be involved in the tonic inhibitory activity within the HPA axis, GRs appear to “switch off” cortisol production at times of stress. It seems that MRs are necessary for GC regulation of HPA axis activity during mild stressors but not during stressors that result in a stronger corticosteroid response. It is proposed that the maintenance in corticosteroid homeostasis and the balance in MR-/GR-mediated effects limit vulnerability to stress-related diseases in genetically predisposed individuals (18).
Three different mechanisms of GR resistance have been considered: (I) downregulation secondary to persistent hypercortisolism, (II) a primary alteration in the genetic structure, and (III) a decrease in GR function secondary to alterations in ligand independent pathways (18). It has also been proposed that the balance between MR and GR is an important factor in resilience to stress, and studies suggest that there may be an imbalance in the MR/GR ratio in depression (38). Another possibility (that can happen concomitantly or independent) is the excessive production of corticotropin release factor (CRF) from the hypothalamus. This structure receives fibers from a number of brain areas, notably the brain stem (that receives input from all sensory systems), the prefrontal cortex and the limbic system (i.e., amygdala) (7). The chronic overexpression of CRF in the amygdala is also associated with altered gene expression in the hippocampus and PVN, leading to increased hyperactivity (39). These afferents play an important role in HPA responses to behavioral and emotional stimuli. The elevated CRF secretion will persistently stimulate the HPA axis, leading ultimately to increase in GC levels and to possible mechanisms of dysfunction in GR and MR already described (18). The prolonged exposure to GC has damaging effects in important brain structures, mainly the hippocampus, that are essential for HPA axis restraint, as well as memory consolidation. The role of GC and stress in memory was recently reviewed and linked with potential psychiatric disorders (10).
The serotonin hub
The hippocampus also receives important afferent connections from the Raphe nucleus, the main site of serotonin neuronal projection. Serotonin (5-HT) is a neurotransmitter that has long been implicated in the regulation of emotional responses and behavior in the brain (40). There are, at least, seven known 5-HT receptors spread throughout brain structures, including the hippocampus (15). There is evidence that the expression and function of GR in the hippocampus, mainly MR, is regulated by the stimulation 5-HT receptors. Stressful stimuli increase 5-HT release and turnover in the hippocampus, and it seems reasonable to suggest that some of the changes in mineralocorticoid and GR expression may be mediated, in part at least, by the increase in 5-HT (41). The balance of MR and GR is known to affect brain serotonin systems and may play an etiologic role in serotonin receptor changes, particularly 5-HT1A downregulation, observed in these cases (42) (Figure 1).
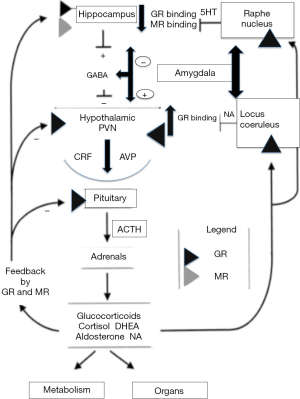
There is evidence that the expression and function of glucocorticosteroid receptors in the hippocampus, mainly MR, is regulated by the stimulation of 5-HT receptors (43). Stressful stimuli increase 5-HT release and turnover in the hippocampus, and it seems reasonable to suggest that some of the changes in mineralocorticoid and GR expression may be mediated, in part at least, by the increase in 5-HT. These findings have led some investigators to propose that postsynaptic 5-HT1A and 5-HT2A receptors have functionally opposing effects, that a disturbed balance of these receptors may be contributing to the pathophysiology of depression, anxiety, and impulsivity, and that restoring this balance is necessary for therapeutic action (42). The balance of MR and GR is known to affect brain serotonin systems and may play an etiologic role in serotonin receptor changes, particularly 5-HT1A downregulation, observed in these cases. Our findings of reduced residual symptoms with spironolactone as an adjunctive therapy in Bipolar patients may be due to a particular characteristic of this group regarding the imbalance of MR and GC action, which can be improved by spironolactone (42). This conclusion is consistent with the possibility that increased 5-HT release in the hippocampus, and spironolactone effects on GC and MR expression, may be of primary importance to the mechanisms by which prior exposure to inescapable stress influences subsequent behavioural and hormonal responses to stressful or anxiogenic stimuli and promotes physiological and behavioural resistance to chronic stress and residual affective symptoms (44). The role of MR antagonist in reducing residual symptoms in bipolar affective disorder patients deserves further investigation through placebo-controlled trials.
There is a complex interaction between corticosteroid receptors and 5HT receptors. The biding potential of 5HT1A receptors, measured by PET-Scan, were recently linked with increased suicidality (45), as well as other serotonin dysfunctions (including elevated TPH2 and abnormal 5HT transporters) in specific areas of the brain. This findings were hypothesized as reflecting low concentrations of available 5HT. CRH receptors were inversely implicated in some brain areas (probably by excess) and HPA axis dysfunction is one of the most consistent findings in a recent review on possible biomarkers for suicide (46). It is possible that the constant interregulation between these systems plays an important role on impulsivity and aggression, key factors in suicide behavior.
Conclusions
It is now widely accepted that ELS plays a key role in the development of psychiatric disorders. Abnormalities in the serotonin system and the HPA axis are also involved in a variety of mental conditions. Studies like Kwan’s (15) help us to shed some light into the complex interaction, constant interplay and possibly bidirectional modulatory effects that these systems have, even in embryo life. They also help us to understand how ELS modulates the capacity of adaptation and how it can produce “scars” that endure for a lifetime. Different areas of very specific subjects of research on different biological mechanisms are now beginning to be integrated, and that advance will hopefully improve our global understanding of mental disorders. It is imperative to find biological substrates and new therapeutic targets and diagnostic models in psychiatric disease and studies like this, and others in basic biological science, play a pivotal role in this challenging and exciting task.
Acknowledgements
Funding: This work was supported by Academy of Medical Sciences/Royal Society, UK and FAPESP (Both to MF Juruena as PI).
Footnote
Conflicts of Interest: MF Juruena has within the last year received honoraria for speaking from GSK, Lundbeck and Pfizer and is a Consultant Psychiatrist at University of Sao Paulo and SLaM (NHS UK); AH Young and AJ Cleare are supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. AJ Cleare has within the last 3 years received honoraria for lectures or consulting from Astra Zeneca, Lundbeck, Livanova & Allergan, and a research grant from Lundbeck. AH Young received honoraria for lectures and advisory boards for all major pharmaceutical companies with drugs used in affective and related disorders. Investigator-initiated studies from AZ, Eli Lilly and Lundbeck. B Agustini has no conflicts of interest to declare.
References
- Juruena MF, Marques AH, Mello AF, et al. A paradigm for understanding and treating psychiatric illness. Rev Bras Psiquiatr 2007;29 Suppl 1:S1-2. [Crossref] [PubMed]
- King SL, Hegadoren KM. An integrative science approach: value added in stress research. Nurs Health Sci 2006;8:114-9. [Crossref] [PubMed]
- Ray O. The revolutionary health science of psychoendoneuroimmunology: a new paradigm for understanding health and treating illness. Ann N Y Acad Sci 2004;1032:35-51. [Crossref] [PubMed]
- Romero LM, Platts SH, Schoech SJ, et al. Understanding stress in the healthy animal - potential paths for progress. Stress 2015;18:491-7. [Crossref] [PubMed]
- van Praag HM. Can stress cause depression? Prog Neuropsychopharmacol Biol Psychiatry 2004;28:891-907. [Crossref] [PubMed]
- Tosevski DL, Milovancevic MP. Stressful life events and physical health. Curr Opin Psychiatry 2006;19:184-9. [Crossref] [PubMed]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 2009;10:397-409. [Crossref] [PubMed]
- de Kloet ER, Karst H, Joëls M. Corticosteroid hormones in the central stress response: quick-and-slow. Front Neuroendocrinol 2008;29:268-72. [Crossref] [PubMed]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci 2005;6:463-75. [Crossref] [PubMed]
- de Quervain D, Schwabe L, Roozendaal B. Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat Rev Neurosci 2017;18:7-19. [Crossref] [PubMed]
- Juruena MF, Cleare AJ, Pariante CM. The hypothalamic pituitary adrenal axis, glucocorticoid receptor function and relevance to depression. Rev Bras Psiquiatr 2004;26:189-201. [Crossref] [PubMed]
- Cowan CS, Callaghan BL, Kan JM, et al. The lasting impact of early-life adversity on individuals and their descendants: potential mechanisms and hope for intervention. Genes Brain Behav 2016;15:155-68. [Crossref] [PubMed]
- Lupien SJ, McEwen BS, Gunnar MR, et al. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 2009;10:434-45. [Crossref] [PubMed]
- Egliston KA, McMahon C, Austin MP. Stress in pregnancy and infant HPA axis function: conceptual and methodological issues relating to the use of salivary cortisol as an outcome measure. Psychoneuroendocrinology 2007;32:1-13. [Crossref] [PubMed]
- Kwan W, Cortes M, Frost I, et al. The Central Nervous System Regulates Embryonic HSPC Production via Stress-Responsive Glucocorticoid Receptor Signaling. Cell Stem Cell 2016;19:370-82. [Crossref] [PubMed]
- Oberlander TF, Grunau R, Mayes L, et al. Hypothalamic-pituitary-adrenal (HPA) axis function in 3-month old infants with prenatal selective serotonin reuptake inhibitor (SSRI) antidepressant exposure. Early Hum Dev 2008;84:689-97. [Crossref] [PubMed]
- Pawluski JL, Brain UM, Underhill CM, et al. Prenatal SSRI exposure alters neonatal corticosteroid binding globulin, infant cortisol levels, and emerging HPA function. Psychoneuroendocrinology 2012;37:1019-28. [Crossref] [PubMed]
- Juruena MF. Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy Behav 2014;38:148-59. [Crossref] [PubMed]
- Avitsur R, Grinshpahet R, Goren N, et al. Prenatal SSRI alters the hormonal and behavioral responses to stress in female mice: Possible role for glucocorticoid resistance. Horm Behav 2016;84:41-9. [Crossref] [PubMed]
- Missault S, Van den Eynde K, Vanden Berghe W, et al. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav Immun 2014;42:138-46. [Crossref] [PubMed]
- Ronovsky M, Berger S, Zambon A, et al. Maternal immune activation transgenerationally modulates maternal care and offspring depression-like behavior. Brain Behav Immun 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Reul JM, Collins A, Saliba RS, et al. Glucocorticoids, epigenetic control and stress resilience. Neurobiol Stress 2014;1:44-59. [Crossref] [PubMed]
- Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci 2014;17:89-96. [Crossref] [PubMed]
- Rodgers AB, Morgan CP, Bronson SL, et al. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 2013;33:9003-12. [Crossref] [PubMed]
- Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol 2005;20:345-52. [Crossref] [PubMed]
- Hoek HW, Brown AS, Susser E. The Dutch famine and schizophrenia spectrum disorders. Soc Psychiatry Psychiatr Epidemiol 1998;33:373-9. [Crossref] [PubMed]
- Veenendaal MV, Painter RC, de Rooij SR, et al. Transgenerational effects of prenatal exposure to the 1944-45 Dutch famine. BJOG 2013;120:548-53. [Crossref] [PubMed]
- Bernstein DP, Stein JA, Newcomb MD, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 2003;27:169-90. [Crossref] [PubMed]
- Grandjean P, Heindel JJ. In utero and early-life conditions and adult health and disease. N Engl J Med 2008;359:1523; author reply 1524. [Crossref] [PubMed]
- Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosom Med 2009;71:805-12. [Crossref] [PubMed]
- Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301:386-9. [Crossref] [PubMed]
- Sorenson AN, Sullivan EC, Mendoza SP, et al. Serotonin transporter genotype modulates HPA axis output during stress: effect of stress, dexamethasone test and ACTH challenge. Transl Dev Psychiatry 2013;1:21130. [Crossref] [PubMed]
- van der Doelen RH, Deschamps W, D'Annibale C, et al. Early life adversity and serotonin transporter gene variation interact at the level of the adrenal gland to affect the adult hypothalamo-pituitary-adrenal axis. Transl Psychiatry 2014;4:e409. [Crossref] [PubMed]
- Juruena MF, Pariante CM, Papadopoulos AS, et al. Prednisolone suppression test in depression: prospective study of the role of HPA axis dysfunction in treatment resistance. Br J Psychiatry 2009;194:342-9. [Crossref] [PubMed]
- Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry 2012;169:141-51. [Crossref] [PubMed]
- Nemeroff CB, Heim CM, Thase ME, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A 2003;100:14293-6. [Crossref] [PubMed]
- Williams LM, Debattista C, Duchemin AM, et al. Childhood trauma predicts antidepressant response in adults with major depression: data from the randomized international study to predict optimized treatment for depression. Transl Psychiatry 2016;6:e799. [Crossref] [PubMed]
- Baes Cv, Martins CM, Tofoli SM, et al. Early Life Stress in Depressive Patients: HPA Axis Response to GR and MR Agonist. Front Psychiatry 2014;5:2. [Crossref] [PubMed]
- Flandreau EI, Ressler KJ, Owens MJ, et al. Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology 2012;37:27-38. [Crossref] [PubMed]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin:news from mouse molecular genetics. Nat Rev Neurosci 2003;4:1002-12. [Crossref] [PubMed]
- Porter RJ, Gallagher P, Watson S, et al. Corticosteroid-serotonin interactions in depression: a review of the human evidence. Psychopharmacology (Berl) 2004;173:1-17. [Crossref] [PubMed]
- Juruena MF, Gama CS, Berk M, et al. Improved stress response in bipolar affective disorder with adjunctive spironolactone (mineralocorticoid receptor antagonist): case series. J Psychopharmacol 2009;23:985-7. [Crossref] [PubMed]
- Semont A, Fache M, Ouafik L, et al. Effect of serotonin inhibition on glucocorticoid and mineralocorticoid expression in various brain structures. Neuroendocrinology 1999;69:121-8. [Crossref] [PubMed]
- Deakin JF, Graeff FG. 5-HT and mechanisms of defence. Author's response. J Psychopharmacol 1991;5:339-41. [Crossref] [PubMed]
- Oquendo MA, Galfalvy H, Sullivan GM, et al. Positron Emission Tomographic Imaging of the Serotonergic System and Prediction of Risk and Lethality of Future Suicidal Behavior. JAMA Psychiatry 2016;73:1048-55. [Crossref] [PubMed]
- Oquendo MA, Sullivan GM, Sudol K, et al. Toward a biosignature for suicide. Am J Psychiatry 2014;171:1259-77. [Crossref] [PubMed]
Cite this article as: Juruena MF, Agustini B, Cleare AJ, Young AH. A translational approach to clinical practice via stress-responsive glucocorticoid receptor signaling. Stem Cell Investig 2017;4:13.


