Novel methods for the treatment of liver fibrosis using in vivo direct reprogramming technology
Liver fibrosis caused by an excessive accumulation of extracellular matrix proteins in the liver has been recognized as one of the irreversible chronic liver failures. Patients of liver cirrhosis have dreamed of the recovery from the fibrosis for a long time, and recently, it was reported that regression of liver fibrosis could be induced by elimination of hepatitis B virus (HBV) and hepatitis C virus (HCV) from the liver (1,2). However, because regression of liver fibrosis occurs slowly and incompletely, it is still important to continue the development of more efficient therapeutic methods for the treatment of liver fibrosis.
Human induced pluripotent stem (iPS) cells have a potential for providing an epoch-making method in the realm of the regenerative medicine (3). Indeed, several protocols for inducing hepatocyte differentiation from iPS cells have been reported (4). These iPS cell-derived hepatocyte-like cells are expected to be useful in clinical applications towards liver diseases, including liver fibrosis. Indeed, iPS cell-derived hepatocyte-like cells could engraft into the tissue of fibrotic liver and partially support liver function (5,6). Meanwhile, hepatocyte-like cells can also be generated directly from mouse and human skin-derived fibroblasts in vitro (7-10). These cells are called induced hepatocyte-like (iHep) cells and generated by inducing direct reprogramming of fibroblasts using defined transcription factors. Similar to iPS cell-derived hepatocyte-like cells, iHep cells are also expected to be a potent cell source to improve liver function in fibrosis. However, since iHep cells have never been transplanted into the liver of animal models for liver fibrosis, it remains unknown whether iHep cells can engraft into the fibrotic liver tissues and show therapeutic effects on liver fibrosis by inducing its regression.
In contrast to in vitro induction of iHep cells, Song et al. and Rezvani et al. recently enabled induction of direct reprograming of liver-resident myofibroblasts into iHep cells in vivo (11,12), which would be new methods for treating liver fibrosis (Table 1). Song et al. demonstrated that forced expression of four transcription factors, such as Foxa3, Gata4, Hnf1α, and Hnf4α, enabled generation of iHep cells from myofibroblasts in the fibrotic mouse liver. Overexpression of the transcription factors in myofibroblasts was carried out with an adenovirus vector in which all four transcription factors could be expressed from a polycistronic transgene cassette. Also, the viruses were modified to bind specifically to the p75 neurotrophin receptor (p75NTR) located on myofibroblasts and hepatic stellate cells that transform into myofibroblasts in the injured liver. Thus, the modified adenovirus had a tropism to myofibroblasts, while transduction was also observed in less than 0.05% of hepatocytes, but not in Kupffer cells, endothelial cells, bile duct cells, and liver stem/progenitor cells. Although p75NTR was also expressed in tissues other than the fibrotic liver, iHep cells were not observed in the brain, heart, lung, and kidney. Following injection of the adenovirus into the fibrotic liver, less than 20% of myofibroblasts was transduced. The percentages of the in vivo generated iHep cells in total hepatocytes ranged from 0.2% to 1.2%. Because the percentages of myofibroblasts residing in the fibrotic liver was from 15.4% to 21.7%, the reprograming efficiency was estimated at less than 4%. Meanwhile, Rezvani et al. used adeno-associated virus serotype 6 (AAV6) vectors to overexpress the other set of transcription factors in myofibroblasts. This study showed that 23.6%±5.0% of myofibroblasts in the fibrotic liver was transduced by AAV6, while 7.9%±7.15% of Kupffer cells and 1.2%±0.53% of hepatocytes were also infected with the virus. In contrast, transduced bile duct cells and endothelial cells were not observed. Rezvani et al. used six individual AAV6 vectors that expressed genes encoding the transcription factors, Foxa1, Foxa2, Foxa3, Gata4, Hnf1α, or Hnf4α, respectively, to induce direct generation of iHep cells in vivo. The percentage of myofibroblast-derived iHep cells in total hepatocytes was less than 0.87%, while Kupffer cell-derived iHep cells were rare (<0.01%). Although hepatocyte markers were also expressed weakly in the skeletal muscle, heart, and spleen, these cells did not show the morphological properties of hepatocytes. Both above methods described in two papers resulted in a similar low efficiency of the conversion of myofibroblast into iHep cells (less than 1% of total hepatocytes). However, induction of iHep cells from myofibroblasts in vivo could contribute to reducing the amount of fibrotic tissues and mitigating liver damage in several mouse models of liver fibrosis, although it was not examined whether myofibroblast-derived iHep cells could support liver functions, including protein synthesis and detoxification. Nevertheless, successful regression of liver fibrosis by inducing conversion of myofibroblasts into iHep cells would enable early treatment of a number of patients having fibrotic liver tissues, which could decrease the incidence of liver cirrhosis, cancer, and in turn functional impairment.
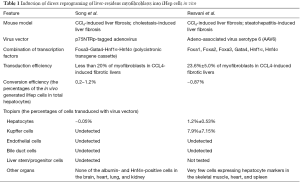
Full table
The findings presented in the studies of Song et al. and Rezvani et al. raised a fundamental question: what improved the liver failure after induction of direct reprogramming of myofibroblasts? The low efficiency of myofibroblast conversion into iHep cells indicates that only a small number of hepatocyte-like cells increased after induction of in vivo direct reprogramming. Thus, the mitigation of liver damage might be due to a reduction of extracellular matrix proteins produced by myofibroblasts. If it is true, the regression of fibrosis might be effective for protecting hepatocytes from liver injury, and dysfunction of the liver might be attenuated following conversion of only a small number of myofibroblasts into iHep cells. Alternatively, there is another possibility that myofibroblast-derived iHep cells could improve the function of “unhealthy hepatocytes” residing in the fibrotic liver. Although Song et al. showed that myofibroblast-derived iHep cells were unable to suppress activation of hepatic stellate cells in vitro, it remains unclear whether iHep cells have a protective effect for damaged hepatocytes in vivo. Interestingly, transplanted iHep cells could promote proliferation of recipient hepatocytes in a mouse model of CCl4-induced acute liver failure (13). Thus, it is suggested that newly generated iHep cells can mitigate the damage of hepatocytes in the fibrotic liver. If the synergistic effect of a decrease and an increase of fibrotic tissues and functional cells, respectively, has a potential to ameliorate fibrosis, this new technology of in vivo direct reprogramming will be useful to treat not only liver fibrosis, but also many other irreversible fibrotic diseases in the lung, kidney, etc., which have no universal treatment as yet.
In addition, there is another question: is this new technology able to prevent liver carcinogenesis developing from liver fibrosis? A long-term chronic inflammation in the fibrotic liver tissues allows accumulation of genomic mutations and epigenetic alterations, resulting in liver carcinogenesis. In fact, it is known that patients of liver cirrhosis have a risk of a later development of liver cancer, even after eliminating HCV from the liver (1). Thus, it might be difficult to prevent liver carcinogenesis even after induction of in vivo direct reprograming of myofibroblasts into iHep cells in liver fibrosis. Because prevention of liver carcinogenesis is an ultimate goal for the treatment of liver fibrosis, it is necessary to develop other clinical approaches, for example, transplantation of liver tissues/organs generated artificially from iHep cells or iPS cell-derived hepatocyte-like cells.
The technology of in vivo direct reprogramming opened up a new strategy for the treatment of liver fibrosis. Further improvement in the safety and the efficiency will facilitate the clinical application of such a new technology, which could make a dramatic progress in the treatment of liver fibrosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- George SL, Bacon BR, Brunt EM, et al. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology 2009;49:729-38. [Crossref] [PubMed]
- Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013;381:468-75. [Crossref] [PubMed]
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861-72. [Crossref] [PubMed]
- Sampaziotis F, Segeritz CP, Vallier L. Potential of human induced pluripotent stem cells in studies of liver disease. Hepatology 2015;62:303-11. [Crossref] [PubMed]
- Liu H, Kim Y, Sharkis S, et al. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci Transl Med 2011;3:82ra39. [Crossref] [PubMed]
- Asgari S, Moslem M, Bagheri-Lankarani K, et al. Differentiation and transplantation of human induced pluripotent stem cell-derived hepatocyte-like cells. Stem Cell Rev 2013;9:493-504. [Crossref] [PubMed]
- Huang P, He Z, Ji S, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 2011;475:386-9. [Crossref] [PubMed]
- Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 2011;475:390-3. [Crossref] [PubMed]
- Du Y, Wang J, Jia J, et al. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell 2014;14:394-403. [Crossref] [PubMed]
- Huang P, Zhang L, Gao Y, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell 2014;14:370-84. [Crossref] [PubMed]
- Rezvani M, Español-Suñer R, Malato Y, et al. In Vivo Hepatic Reprogramming of Myofibroblasts with AAV Vectors as a Therapeutic Strategy for Liver Fibrosis. Cell Stem Cell 2016;18:809-16. [Crossref] [PubMed]
- Song G, Pacher M, Balakrishnan A, et al. Direct Reprogramming of Hepatic Myofibroblasts into Hepatocytes In Vivo Attenuates Liver Fibrosis. Cell Stem Cell 2016;18:797-808. [Crossref] [PubMed]
- Lim KT, Lee SC, Gao Y, et al. Small Molecules Facilitate Single Factor-Mediated Hepatic Reprogramming. Cell Rep 2016. [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Goya T, Suzuki A. Novel methods for the treatment of liver fibrosis using in vivo direct reprogramming technology. Stem Cell Investig 2016;3:92.


