Wilms tumor 1 peptide vaccination after hematopoietic stem cell transplant in leukemia patients
Leukemia patients who have not been cured by drug therapy alone require allogeneic hematopoietic stem cell transplantation (HSCT). Although the prognosis for leukemia patients who underwent allogeneic HSCT has greatly improved, relapse remains a major concern. In allogeneic HSCT, chemotherapy-resistant leukemia cells can be eliminated as a result of immunologic rejection of recipient leukemia cells by donor T cells, known as the graft-versus-leukemia (GVL) effect (1). Allogeneic HSCT is an immunotherapy that exploits the allo-immune response of donor immune cells against leukemia cells. Thus, enhancement of this response is the most straightforward strategy for preventing relapse after allo HSCT, as indicated by recent success of checkpoint antibody therapy for post-HSCT relapse (2). However, it is not easy to clearly separate the GVL effect from graft-versus-host disease (GVHD). For this purpose, cancer vaccination represents a promising strategy. In this review, we will summarize the results of clinical trials of cancer vaccines for hematological malignancies, mainly after allogeneic HSCT. We also discuss the advantages of the immunological milieu after allogeneic HSCT for immunotherapy and the future prospects for this field.
Vaccines for hematological malignancies
In several types of cancers, allogeneic tumor cells expressing granulocyte-macrophage colony-stimulating factor (GM-CSF) (GVAX) were tested as vaccine (3-5). For example, K562 cells expressing GM-CSF were used as vaccine for CML patients. In some patients, the abundance of CML cells decreased after immunotherapy, and this effect was associated with the induction of high-titer IgG antibodies against multiple leukemia-associated antigens (LAAs) (6). In another trial by Borrello et al., pre-auto HSCT patients were immunized with autologous leukemia cells mixed with GM-CSF-secreting K562 cells (7). A decrease in Wilms tumor 1 (WT1) transcripts in blood was noted in 69% of patients after immunotherapy, and was associated with longer 3-year relapse-free survival (61% in the immunized group vs. 0% in the non-immunized group). For successful use of tumor cell vaccines, the usage of appropriate adjuvant is essential. Recently, Gibbins et al. reported that an intravenously administered vaccine consisting of irradiated leukemia cells loaded with the natural killer T (NKT)-cell agonist alpha-galactosylceramide (alpha-GalCer) was effective in a mouse leukemia model (8).
Dendritic cell (DC)-based vaccines represent another effective strategy. For example, leukemic DCs generated from peripheral blood of CML patients were used as a vaccine and shown to elicit a tumor-reactive T cells response (9). DC fusions with leukemia cells or DCs loaded with tumor cell lysates also induced a potent tumor immune response (10). In addition, vaccination with WT1 mRNA-electroporated DCs induces molecular remission in AML patients (11).
Several kinds of peptide vaccines have been developed. For example, LAA-derived peptides (12) and DNA (13) have been used as vaccines in combination with adjuvants. BCR-ABL for Philadelphia-chromosome–positive leukemia (14), and over-or aberrantly expressed LAAs such as proteinase 3 (PR3) (15,16), WT1 (17,18), PRAME (19), have been tested as targets. Among these targets, WT1 is over-expressed in most types of acute and chronic leukemia, and is thus one of the most promising targets for immunotherapy against leukemia. We and other researchers demonstrated the safety and immunogenicity of WT1 peptide vaccine for patients with leukemia (20-24). Several groups have confirmed induction of immune responses by vaccination with WT1 peptide in patients with AML (25-27). In these studies, not only immunological responses but also clinical responses (including stable disease and reduced expression of tumor markers) were observed in a substantial portion of evaluable patients. Regression of minimal residual disease in leukemia patients who received repeated vaccination with the WT1-derived peptide has also been reported (28). Recently, Brayer et al. reported that WT1 peptide vaccine was well-tolerated in leukemia patients, and that clinical benefits were observed in several patients (29).
Taken together, these findings indicate that tumor cell lysates, DCs, and peptide vaccines have the potential to induce T-cell responses. However, it remains unclear whether these vaccines can significantly benefit patients, and therefore they must be tested in randomized clinical trials. Soon, the results of several ongoing clinical trials using peptide vaccines will reveal the efficacy of this approach.
The post-allogeneic transplant period provides a unique platform for vaccination
The immunological milieu after allogeneic HSCT is suitable for the use of cancer vaccines, as described below (Figure 1).
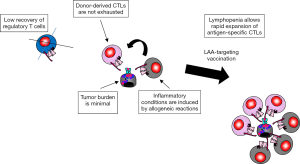
Tumor burden is minimal after allogeneic HSCT
The ratio between target and effector cells is important for immune-mediated cancer therapy. In many cases of advanced disease, the numbers of antigen-specific cytotoxic T cells (CTLs) elicited by immunotherapy may not be sufficient to compete with large numbers of tumor cells. Several clinical studies demonstrated that of cancer vaccines had significant potential to elicit T-cell response, but did not show clinical benefits. Thus, patients with minimal tumor burden might be appropriate targets for immunotherapy. Because high-dose chemotherapy and/or total body irradiation before transplant, along with the GVL effect after transplant, minimize leukemia burden, the post-transplant period is suitable for immunotherapy.
Lymphopenia allows for rapid expansion of antigen-specific CTLs
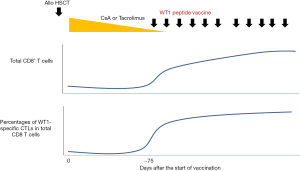
The affinities of T-cell receptors (TCRs) to LAA-derived peptides are low because most LAAs are self antigens. The homeostatic expansion of T cells facilitates and enhances T-cell proliferation in response to low-affinity self-antigens (30). Severe lymphopenia induced after allogeneic HSCT lowers the activation threshold of antigen-specific T cells and promotes thymic-independent homeostatic T-cell proliferation (30-34). In the first few months after transplantation, the T-cell repertoire is oligoclonal, with skewing toward host (35), tumor, and viral antigens (34), although global immune functions remain severely impaired. Reconstitution of the T-cell compartment in lymphopenic hosts is regulated by peptides occupying MHC class I and II molecules at the time of T-cell recovery (36). Therefore, there may be an opportunity to skew the T-cell repertoire in a favorable direction by engaging the available MHC class I and class II molecules with peptides of specific interest. Thus, vaccination with LAA-peptides that can bind to MHC class I and class II molecules may induce specific expansion of LAA-reactive T cells. These considerations imply that the first few months after transplantation might be an appropriate period for vaccination with LAA-derived peptides to induce T-cell responses. In fact, in our clinical trial of WT1 peptide vaccine, efficient expansion of WT1-specific CD8 CTLs was observed along with recovery of total CD8 T cells following administration of the WT1 vaccine (Figure 2).
Donor-derived antigen-specific CTLs are not exhausted
T cells isolated from human tumors, as well as experimental tumor models, share many phenotypic and functional characteristics with those of exhausted T cells in cases of chronic infection. Tumor-infiltrating CD8 T lymphocytes (TILs) are deficient in production of effector cytokines and express inhibitory receptors such as PD-1, LAG-3, 2B4, TIM-3, and CTLA-4. In addition, changes in signaling pathways similar to those observed in exhausted T cells in chronic infection models have been observed in TILs (37). Based on these findings, it is commonly assumed that T cells in progressive cancers are in an exhausted state due to a high tumor-antigen load and the immunosuppressive factors present in the tumor microenvironment (38). By contrast, donor-derived LAA-specific T cells in allogeneic HSCT have not been extensively exposed to LAAs, and consequently are less likely to be exhausted.
Inflammatory conditions are induced by allogeneic reactions
The GVL effect in syngeneic HSCT is not as strong as that in allogeneic HSCT, suggesting that LAAs alone are not sufficient to induce GVL after allogeneic HSCT. In allogeneic HSCT, inflammatory conditions caused by the GVL and GVHD responses against alloantigens may activate antigen-presenting cells and facilitate T-cell response to LAAs. Such inflammatory conditions are advantageous for vaccination therapies.
Unbalanced recovery of regulatory and effector T cells
The balance between the recovery of CD4 regulatory T cells and effector T cells is important for the development and maintenance of immune tolerance after allogeneic HSCT. Recently, Alho et al. (39) reported reduced recovery of Treg cells after allogeneic HSCT. Specifically, they analyzed 107 adult patients who received allogeneic HSCT after reduced-intensity conditioning. CD8 T cells recovered more rapidly than either CD4 Tregs or conventional CD4 T cells. Moreover, T-cell proliferation was skewed in favor of conventional CD4T and CD8 T cells, especially 6 to 12 months after HSCT. These results also suggest that the time period after allogeneic HSCT is advantageous for cancer vaccines.
Cancer vaccination after allogeneic HSCT
In patients with acute and chronic leukemia after allogeneic HSCT, there is an inverse relationship between the number of circulating T cells directed against LAAs and that of residual leukemia cells (40-42). This observation suggests that GVL effects might be further enhanced by vaccination targeting LAAs (43). However, only a few clinical trials of vaccination have been conducted in the patients who had undergone HSCT, and at least to our knowledge, all of these trials were pilot or phase I studies. In addition, the vaccination strategies in these trials were variable, with tumor cells, DCs, or peptides used as vaccines.
Tumor cell vaccine
A recipient-derived tumor cell vaccine was used in leukemia patients, including some who had undergone allogeneic HSCT (44). Ten patients with high-risk acute myeloid (n=4) or lymphoblastic (n=6) leukemia in cytological remission [after allogeneic HSCT (n=9) or chemotherapy alone (n=1)] were enrolled in this study. The vaccine consisted of leukemic blasts mixed with skin fibroblasts transduced with adenoviral vectors expressing human IL-2 and hCD40L. Immunization produced a 10- to 890-fold increase in the frequencies of major histocompatibility complex (MHC)-restricted T cells reactive against recipient-derived blasts. Eight patients remained disease-free for 27–62 months after treatment. In another study, high-risk acute myeloid leukemia or myelodysplasia patients were immunized with irradiated, autologous, GM-CSF-secreting tumor cells soon after allogeneic non-myeloablative HSCT (45). Although the frequencies of acute and chronic GVHD did not increase, 9 of the 10 subjects who completed the vaccination schedule attained long-lasting complete remissions during a median follow-up of 26 months. Burkhardt et al. performed a prospective clinical trial to evaluate whether vaccination with whole leukemia cells soon after transplantation facilitates the expansion of leukemia-reactive T cells, thereby enhancing antitumor immunity (46). This vaccine consisted of irradiated autologous tumor cells mixed with GM-CSF-secreting bystander cells. Eighteen patients with advanced chronic lymphocytic leukemia (CLL) received the vaccine, starting between 30 and 45 days after transplantation. The estimated 2-year progression-free and overall survival rates of vaccinated subjects were 82% and 88%, respectively. CD8+ T cells from vaccinated patients consistently reacted against autologous tumor, but not allogeneic antigen-bearing recipient cells. All of these trials using tumor cell vaccines showed promising results, and at a minimum demonstrated the safety of this approach. The benefit to patient survival will have to be further evaluated in randomized studies. Unfortunately, the procedures for generating tumor cell vaccines are complex and difficult to standardize.
DC vaccine
A pilot study of vaccination with DCs pulsed with idiotype (Id)-derived peptide was performed in multiple myeloma (MM) patients after allogeneic HSCT (47). Following reduced intensity conditioning allogeneic HSCT and failure of rescue therapy with donor lymphocyte infusion (DLI) or chemotherapy, four patients underwent vaccination with Id-derived peptide- and keyhole limpet hemocyanin (KLH)-pulsed donor-derived DCs after disease relapse/progression. An Id-KLH-specific T-cell response was detected in vitro. Two patients exhibited a transient response. However, three patients, including one responder ultimately suffered disease progression. In addition, DC vaccination of an AML patient after allogeneic HSCT was reported (48). The AML patient underwent vaccination with WT1 peptide- and KLH-pulsed donor-derived DCs to treat relapse after allogeneic HSCT. In this patient, leukemia gradually progressed despite vaccination, and immune responses to the naive antigen KLH, but not to WT1, were detected. These studies proved the safety of DC vaccination after allogeneic HSCT; however, the clinical benefits were limited for patients with advanced disease. The ability of DC vaccines to prevent relapse in patients with disease remission after HSCT needs to be tested in future studies.
Peptide vaccine
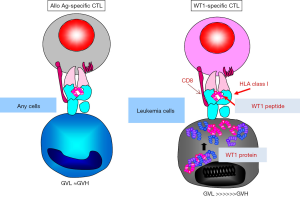
Several reports suggested that T-cell response to LAAs such as PR3 or WT1 is correlated with reduction of leukemia burden (41,49). Thus, the GVL effect may be boosted using peptide vaccines that target LAAs. Especially in the post–allotransplant setting, WT1-specific CTLs are excellent effector cells of GVL because WT1 expression in normal tissues is very limited. In other words, we can separate the effects of GVL from those of GVHD by using WT1-specific CTLs as effector cells (Figure 3). Hashii et al. reported the results of WT1-derived peptide vaccination for three pediatric leukemia patients with high risk for relapse after allogeneic HSCT (50). These patients underwent weekly injections of HLA-A*2402-restricted, 9-mer-modified WT1 peptide (a.a. 235–243, CYTWNQML) emulsified in Montanide ISA 51 adjuvant. Vaccinations were started between 41 and 173 days post-SCT. Reduced WT1 mRNA levels in BM and elevated numbers of WT1-specific CTLs were observed in all three cases. Two of three cases have remained in CR 33.5 and 40.3 months after HSCT. In one case, disease recurred on day 201 after the start of vaccination, and the frequencies of WT1-specific CTLs in peripheral blood CD8+ T cells increased to 0.85%. In that case, HLA expression on leukemic cells was lost, implying immunological escape of the leukemic cells. Our group reported the results of a phase I study of WT1 peptide vaccination in adult hematological malignancy patients with recurrent disease or high risk factors for relapse after HSCT (51). The study design was almost identical to that of Hashii et al. Of the nine patients enrolled, three were in complete remission (CR), two were in molecular relapse, and the remaining four were suffering from hematological relapse. We confirmed that an antigen-specific CTL response could be elicited even in patients who were given immunosuppressive drugs such as tacrolimus or prednisolone. Three patients in molecular CR remained in CR for 2 years, whereas disease gradually progressed in the patients with hematologically relapsed disease. Two patients with molecular residual disease achieved CR after the start of vaccination.
These reports show that (I) tumor cell vaccines, DC vaccines, and peptide vaccines can induce antigen-specific T cell response in post allogeneic HSCT patients, even in patients who are given immunosuppressive drugs; (II) the results of trials using tumor cell vaccine and peptide vaccine are promising; and (III) it may be better to test the effect of vaccination on the prevention of relapse rather than the treatment of hematological relapse that has already occurred. To evaluate whether vaccination prevents disease relapse and benefits patients, well-controlled randomized trials should be conducted.
Future directions
Vaccination therapy has the potential to enhance the GVL effect, and thus represents a promising tool for preventing relapse after allogeneic HSCT. To develop more effective vaccination therapies, several trials are being conducted to improve the effect of vaccination after allogeneic HSCT.
Combination with DLI
DLI is widely used as a treatment for relapse after allogeneic HSCT (52). Because the effectors of DLI include LAAs-specific CTLs, it is reasonable to expand this population by vaccination after DLI. In addition, Bachireddy et al. recently reported an interesting effect of DLI (53). They analyzed the characteristics of cells from 29 patients categorized according to their response to DLI, and found that gene expression profiles before DLI showed evidence of “exhaustion” in T cells specifically in the marrow of responders; specifically, the response after DLI was associated with down-regulation of the pertinent genes. These results suggest that infused donor CD4 T cells might eliminate recipient leukemia cells by reversing exhaustion in donor CD8 T cells that had previously infiltrated the marrow.
Activation of CD4 helper T cells
CD4 T helpers are not required for the primary expansion and differentiation of CD8 T cytotoxic effectors, but they are necessary for the secondary expansion of these effectors. The importance of CD4 helper T cells in tumor immunity has been highlighted by several authors. Tran et al. recently reported convincing evidence from a clinical study showing that CD4 helper T cells play essential roles in tumor immunity (54). In particular, they showed that tumor-infiltrating lymphocytes (TILs) from a patient with metastatic cholangiocarcinoma contained CD4+ T cells that recognized a mutation in Erbb2-interacting protein, and that adoptively transferred mutation-reactive CD4 T cells induced tumor regression. To activate WT1-specific CD4 T cells along with CD8 CTLs, a polyvalent vaccine consisting of longer synthetic peptides was developed to induce stronger WT1-specific CD8+ and CD4+T-cell responses across several HLA types, as well as support long-lasting immunity (27). Our group also developed a strategy for enhancing WT1-specific CD4 T cell response (55,56). The clinical benefits of CD4-directed vaccine after allogeneic HSCT have not been evaluated, and should be tested in the near future. Meanwhile, the influence of CD4 activation on GVHD should be assessed very carefully.
Combination with immune checkpoint antibody
Exhaustion of CTLs in advanced disease has been reported in leukemia models. However, the recent success of checkpoint antibodies clearly demonstrates that exhausted status of CTLs is reversible. Therefore, it would be reasonable to investigate the synergistic effect of vaccination and checkpoint antibodies. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) is a key negative regulator of T cell activation and proliferation. Bashey et al. conducted a phase I clinical trial of ipilimumab, an antagonist for CTLA-4, in patients with relapsed malignancy following allogeneic HSCT (57,58). In that study, only a single infusion was administered to patients, primarily to measure the effect of ipilimumab on the sizes of T-cell subpopulations. A more recent study reported strong efficacy of anti-CTLA4 mAb therapy for disease relapse after allogeneic HSCT (2). In that trial, patients with relapsed hematologic cancer after allogeneic HSCT received an infusion of ipilimumab. Among 22 patients who received a dose of 10 mg/kg, 5 (23%) had a complete response and two (9%) had a partial response. Furthermore, four of the responding patients had a durable response lasting more than 1 year. Responders exhibited infiltration of cytotoxic CD8+ T cells and decreased activation of regulatory T cells. Immune-related adverse events, including one death, were observed in 6 patients (21%), and severe GVHD in 4 (14%). Vaccination is expected to have a synergistic effect with other checkpoint antibodies, including anti-programmed cell death 1 (PD1) or PDL1 mAbs; however, the influence of these antibodies on GVHD should be assessed very carefully.
Conclusions
To prevent relapse after HSCT, cancer vaccines targeting LAAs such as WT1 is a promising strategy. Randomized clinical trials will reveal the efficacy of this approach in the near future. It is also important to consider the combination of cancer vaccine with checkpoint antibodies.
Acknowledgements
The authors wish to thank all staffs of the Departments of Hematology and Oncology in Osaka University, Osaka City University, and Kyoto University for their outstanding management of the patients. The authors also would like to thank Dr. Yuji Heike (National Cancer Center of Japan) for the fruitful discussion.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Weiden PL, Flournoy N, Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med 1979;300:1068-73. [Crossref] [PubMed]
- Davids MS, Kim HT, Bachireddy P, et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N Engl J Med 2016;375:143-53. [Crossref] [PubMed]
- Nemunaitis J, Jahan T, Ross H, et al. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther 2006;13:555-62. [Crossref] [PubMed]
- Higano CS, Corman JM, Smith DC, et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer 2008;113:975-84. [Crossref] [PubMed]
- van den Eertwegh AJ, Versluis J, van den Berg HP, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 2012;13:509-17. [Crossref] [PubMed]
- Qin L, Smith BD, Tsai HL, et al. Induction of high-titer IgG antibodies against multiple leukemia-associated antigens in CML patients with clinical responses to K562/GVAX immunotherapy. Blood Cancer J 2013;3:e145. [Crossref] [PubMed]
- Borrello IM, Levitsky HI, Stock W, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting cellular immunotherapy in combination with autologous stem cell transplantation (ASCT) as postremission therapy for acute myeloid leukemia (AML). Blood 2009;114:1736-45. [Crossref] [PubMed]
- Gibbins JD, Ancelet LR, Weinkove R, et al. An autologous leukemia cell vaccine prevents murine acute leukemia relapse after cytarabine treatment. Blood 2014;124:2953-63. [Crossref] [PubMed]
- Choudhury A, Gajewski JL, Liang JC, et al. Use of leukemic dendritic cells for the generation of antileukemic cellular cytotoxicity against Philadelphia chromosome-positive chronic myelogenous leukemia. Blood 1997;89:1133-42. [PubMed]
- Duncan C, Roddie H. Dendritic cell vaccines in acute leukaemia. Best Pract Res Clin Haematol 2008;21:521-41. [Crossref] [PubMed]
- Van Tendeloo VF, Van de Velde A, Van Driessche A, et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms' tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci U S A 2010;107:13824-9. [Crossref] [PubMed]
- Rezvani K. Peptide vaccine therapy for leukemia. Int J Hematol 2011;93:274-80. [Crossref] [PubMed]
- Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer 2008;8:108-20. [Crossref] [PubMed]
- Molldrem J, Dermime S, Parker K, et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood 1996;88:2450-7. [PubMed]
- Molldrem JJ, Clave E, Jiang YZ, et al. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units. Blood 1997;90:2529-34. [PubMed]
- Molldrem JJ, Lee PP, Wang C, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med 2000;6:1018-23. [Crossref] [PubMed]
- Gao L, Xue SA, Hasserjian R, et al. Human cytotoxic T lymphocytes specific for Wilms' tumor antigen-1 inhibit engraftment of leukemia-initiating stem cells in non-obese diabetic-severe combined immunodeficient recipients. Transplantation 2003;75:1429-36. [Crossref] [PubMed]
- Oka Y, Udaka K, Tsuboi A, et al. Cancer immunotherapy targeting Wilms' tumor gene WT1 product. J Immunol 2000;164:1873-80. [Crossref] [PubMed]
- Quintarelli C, Dotti G, De Angelis B, et al. Cytotoxic T lymphocytes directed to the preferentially expressed antigen of melanoma (PRAME) target chronic myeloid leukemia. Blood 2008;112:1876-85. [Crossref] [PubMed]
- Oka Y, Tsuboi A, Taguchi T, et al. Induction of WT1 (Wilms' tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci U S A 2004;101:13885-90. [Crossref] [PubMed]
- Oka Y, Tsuboi A, Murakami M, et al. Wilms tumor gene peptide-based immunotherapy for patients with overt leukemia from myelodysplastic syndrome (MDS) or MDS with myelofibrosis. Int J Hematol 2003;78:56-61. [Crossref] [PubMed]
- Gao L, Bellantuono I, Elsasser A, et al. Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood 2000;95:2198-203. [PubMed]
- Gaiger A, Reese V, Disis ML, et al. Immunity to WT1 in the animal model and in patients with acute myeloid leukemia. Blood 2000;96:1480-9. [PubMed]
- Ohminami H, Yasukawa M, Fujita S. HLA class I-restricted lysis of leukemia cells by a CD8(+) cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood 2000;95:286-93. [PubMed]
- Rezvani K, Yong AS, Mielke S, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood 2008;111:236-42. [Crossref] [PubMed]
- Keilholz U, Letsch A, Busse A, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood 2009;113:6541-8. [Crossref] [PubMed]
- Maslak PG, Dao T, Krug LM, et al. Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T-cell responses in patients with complete remission from acute myeloid leukemia. Blood 2010;116:171-9. [Crossref] [PubMed]
- Tsuboi A, Oka Y, Kyo T, et al. Long-term WT1 peptide vaccination for patients with acute myeloid leukemia with minimal residual disease. Leukemia 2012;26:1410-3. [Crossref] [PubMed]
- Brayer J, Lancet JE, Powers J, et al. WT1 vaccination in AML and MDS: A pilot trial with synthetic analog peptides. Am J Hematol 2015;90:602-7. [Crossref] [PubMed]
- Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity 1999;11:183-90. [Crossref] [PubMed]
- Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol 2007;19:318-30. [Crossref] [PubMed]
- Clarke SR, Rudensky AY. Survival and homeostatic proliferation of naive peripheral CD4+ T cells in the absence of self peptide:MHC complexes. J Immunol 2000;165:2458-64. [Crossref] [PubMed]
- Cho BK, Rao VP, Ge Q, et al. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med 2000;192:549-56. [Crossref] [PubMed]
- Mackall CL, Bare CV, Granger LA, et al. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol 1996;156:4609-16. [PubMed]
- Michálek J, Collins RH, Hill BJ, et al. Identification and monitoring of graft-versus-host specific T-cell clone in stem cell transplantation. Lancet 2003;361:1183-5. [Crossref] [PubMed]
- Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol 2005;17:183-91. [Crossref] [PubMed]
- Baitsch L, Baumgaertner P, Devevre E, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest 2011;121:2350-60. [Crossref] [PubMed]
- Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol 2014;35:51-60. [Crossref] [PubMed]
- Alho AC, Kim HT, Chammas MJ, et al. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood 2016;127:646-57. [Crossref] [PubMed]
- Morita Y, Heike Y, Kawakami M, et al. Monitoring of WT1-specific cytotoxic T lymphocytes after allogeneic hematopoietic stem cell transplantation. Int J Cancer 2006;119:1360-7. [Crossref] [PubMed]
- Rezvani K, Yong AS, Savani BN, et al. Graft-versus-leukemia effects associated with detectable Wilms tumor-1 specific T lymphocytes after allogeneic stem-cell transplantation for acute lymphoblastic leukemia. Blood 2007;110:1924-32. [Crossref] [PubMed]
- Marijt WA, Heemskerk MH, Kloosterboer FM, et al. Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc Natl Acad Sci U S A 2003;100:2742-7. [Crossref] [PubMed]
- Rezvani K. Posttransplantation vaccination: concepts today and on the horizon. Hematology Am Soc Hematol Educ Program 2011;2011:299-304.
- Rousseau RF, Biagi E, Dutour A, et al. Immunotherapy of high-risk acute leukemia with a recipient (autologous) vaccine expressing transgenic human CD40L and IL-2 after chemotherapy and allogeneic stem cell transplantation. Blood 2006;107:1332-41. [Crossref] [PubMed]
- Ho VT, Vanneman M, Kim H, et al. Biologic activity of irradiated, autologous, GM-CSF-secreting leukemia cell vaccines early after allogeneic stem cell transplantation. Proc Natl Acad Sci U S A 2009;106:15825-30. [Crossref] [PubMed]
- Burkhardt UE, Hainz U, Stevenson K, et al. Autologous CLL cell vaccination early after transplant induces leukemia-specific T cells. J Clin Invest 2013;123:3756-65. [Crossref] [PubMed]
- Bendandi M, Rodriguez-Calvillo M, Inoges S, et al. Combined vaccination with idiotype-pulsed allogeneic dendritic cells and soluble protein idiotype for multiple myeloma patients relapsing after reduced-intensity conditioning allogeneic stem cell transplantation. Leuk Lymphoma 2006;47:29-37. [Crossref] [PubMed]
- Kitawaki T, Kadowaki N, Kondo T, et al. Potential of dendritic-cell immunotherapy for relapse after allogeneic hematopoietic stem cell transplantation, shown by WT1 peptide- and keyhole-limpet-hemocyanin-pulsed, donor-derived dendritic-cell vaccine for acute myeloid leukemia. Am J Hematol 2008;83:315-7. [Crossref] [PubMed]
- Rezvani K, Grube M, Brenchley JM, et al. Functional leukemia-associated antigen-specific memory CD8+ T cells exist in healthy individuals and in patients with chronic myelogenous leukemia before and after stem cell transplantation. Blood 2003;102:2892-900. [Crossref] [PubMed]
- Hashii Y, Sato-Miyashita E, Matsumura R, et al. WT1 peptide vaccination following allogeneic stem cell transplantation in pediatric leukemic patients with high risk for relapse: successful maintenance of durable remission. Leukemia 2012;26:530-2. [Crossref] [PubMed]
- Maeda T, Hosen N, Fukushima K, et al. Maintenance of complete remission after allogeneic stem cell transplantation in leukemia patients treated with Wilms tumor 1 peptide vaccine. Blood Cancer J 2013;3:e130. [Crossref] [PubMed]
- Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995;86:2041-50. [PubMed]
- Bachireddy P, Hainz U, Rooney M, et al. Reversal of in situ T-cell exhaustion during effective human antileukemia responses to donor lymphocyte infusion. Blood 2014;123:1412-21. [Crossref] [PubMed]
- Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014;344:641-5. [Crossref] [PubMed]
- Fujiki F, Oka Y, Tsuboi A, et al. Identification and characterization of a WT1 (Wilms Tumor Gene) protein-derived HLA-DRB1*0405-restricted 16-mer helper peptide that promotes the induction and activation of WT1-specific cytotoxic T lymphocytes. J Immunother 2007;30:282-93. [Crossref] [PubMed]
- Lin Y, Fujiki F, Katsuhara A, et al. HLA-DPB1*05: 01-restricted WT1332-specific TCR-transduced CD4+ T lymphocytes display a helper activity for WT1-specific CTL induction and a cytotoxicity against leukemia cells. J Immunother 2013;36:159-70. [Crossref] [PubMed]
- Bashey A, Medina B, Corringham S, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood 2009;113:1581-8. [Crossref] [PubMed]
- Zhou J, Bashey A, Zhong R, et al. CTLA-4 blockade following relapse of malignancy after allogeneic stem cell transplantation is associated with T cell activation but not with increased levels of T regulatory cells. Biol Blood Marrow Transplant 2011;17:682-92. [Crossref] [PubMed]
Cite this article as: Hosen N, Maeda T, Hashii Y, Tsuboi A, Nishida S, Nakata J, Oji Y, Oka Y, Sugiyama H. Wilms tumor 1 peptide vaccination after hematopoietic stem cell transplant in leukemia patients. Stem Cell Investig 2016;3:90.


