Comment on: Expandable cardiovascular progenitor cells reprogrammed from fibroblasts
Introduction: three pathways to generate new cardiomyocytes (CMs)
Since Takahashi and Yamanaka generated induced pluripotent stem cells (iPSCs) from mice and humans only 10 years ago (1,2), studies of regenerative medicine have been enthusiastically conducted all over the world. By overexpressing four stem cell-specific transcription factors (Oct3/4, Sox2, c-Myc, and Klf4: OSKM, known as the four Yamanaka factors), fibroblasts from mice and humans can be induced to a pluripotent state (1,2). IPSCs have the critically valuable advantage of autotransplantation compared with embryonic stem cells. Much has been learned following the generation of iPSCs. Many terminally differentiated cells (e.g., fibroblasts, CMs, hepatocytes, neural cells, other cells) show substantial cell fate plasticity. These cells can be converted to other types of cells by treatment with defined cytokines and signaling molecules.
In the field of cardiology, iPSC technology provides a novel method to potentially regenerate the damaged myocardium [i.e., following severe heart failure or myocardial infarction (MI)] by directly transplanting CMs derived from iPSCs in situ. However, the full realization of the potential of regenerative therapies with iPSCs will require resolution of many problems. Many laboratories throughout the world, including our own, are working to solve various problems with iPSCs. Overcoming these problems will reveal new methods for utilizing iPSCs in patients with severely damaged myocardium (3-9).
The discovery of iPSCs inspired a new approach that generates specific cell types without needing to transition through a stem cell state. Instead, introducing combinations of lineage-specific factors result in direct reprogramming. In 2010, Ieda et al. reported that cardiomyocyte-like cells can be induced from fibroblasts by transduction of a cocktail of myocardium-specific transcription factors, Gata4, Mef2c, and Tbx5 (GMT). These cardiomyocyte-like cells were named induced cardiomyocytes (iCMs) (10). Efe et al. showed that transient overexpression of OSKM and subsequent exposure to cardiogenic medium components, including bone morphogenetic protein (BMP) 4 and a JAK inhibitor (JI1), convert mouse fibroblasts into spontaneously contracting CMs via a cardiac/cardiovascular progenitor cell (CPC) state with no pluripotent intermediate (11).
Today, three general pathways can be used to generate CMs from fibroblasts (Figure 1):
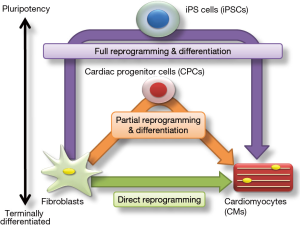
- Full reprogramming of fibroblasts into iPSCs and subsequent cardiac differentiation;
- Partial reprogramming of fibroblasts to CPCs and subsequent differentiation;
- Direct reprogramming of fibroblasts into CMs.
The CMs generated from any of these three pathways can be transplanted into an infarcted or failing heart.
Currently, iPSC generation is the major strategy used to generate CMs. This strategy requires the full reprogramming of fibroblasts into iPSCs and their subsequent differentiation. In other words, this strategy requires the complete initialization to undifferentiated cells from fibroblasts, and differentiation from iPSCs to CMs. Chong et al. reported that directed cardiac differentiation from iPSCs using factors that mimic the developmental signals generates CMs efficiently, and that transplantation of human embryonic stem cell-derived CMs can remuscularize substantial amounts of the infarcted monkey heart, although ventricular arrhythmic complications were also seen (12).
The strategy of producing iCMs, which involves direct reprogramming, could resolve the tendency for tumor formation and shorten the time to generate functional CMs. The new strategy of producing CPCs has the new advantage of self-expandability and differentiation of the three cell types of the heart. In Table 1, we summarize the advantages and disadvantages of the three strategies used to derive CMs from fibroblasts.
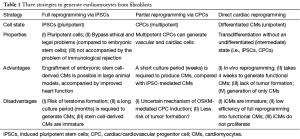
Full table
Reprogrammed CMs can be transplanted into an infarcted or failing heart. The direct injection of cardiac reprogramming transcription factors into the heart may be realized by the direct reprogramming approach, which would not have to rely on the engraftment of iCMs into the heart (13).
The strategy of induced expandable cardiac/cardiovascular progenitor cells (ieCPCs) is novel and important
CPCs are a potentially useful and interesting cellular resource for treating heart disease. There are two reasons about this. First, CPCs are self-expandable, and theoretically, they can be utilized and maintained indefinitely. Second, CPCs can differentiate into the three cell types of the heart, CMs, endothelial cells (ECs), and vascular smooth muscle cells (SMCs). Therefore, many scientists are focused on the generation of CPCs as the third strategy in cardiac regenerative medicine.
Zhang et al. reported induction of CPCs from mouse fibroblasts with combinations of transcription factors and small molecules, and they successfully demonstrated robust expansion of their obtained cell populations in chemically defined conditions (Figure 2) (14). They refined the distinct reprogramming strategy reported by Efe et al. in 2011. First, they transiently overexpressed the four Yamanaka factors (OSKM) in defined combination medium with a JI1, as reported by Efe et al., for 5 days (11). Next, after treatment with JI1 and CHIR99021 (a canonical Wnt activator) for 2 days, Flk-1 and platelet-derived growth factor receptor (PDGF) cells were found after changing to a cocktail of BMP 4, Activin A [the member of the transforming growth factor beta (TGF-β)], CHIR99021 (GSK3 inhibitor), and SU5402 [fibroblast growth factor receptor (FGFR)-specific tyrosine kinase inhibitor] (named BACS) for 14 days. These double-positive cells were called “induced expandable CPCs” (ieCPCs).
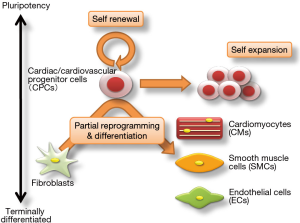
The ieCPCs can be induced to self-renew for 18 passages and self-expand on a large scale to provide cell numbers over 1010 fold in defined conditions including BACS. And these can differentiate into CMs, ECs, or SMCs in specific conditions even after prolonged culture. Interestingly, in non-differentiation conditions including fetal bovine serum, ieCPCs can be converted into Isl1+ progenitor cells.
In vivo, ieCPCs also differentiate into all three lineages (CMs, ECs and SMCs) after transplantation into MI model mice. Transplantation of ieCPCs into an MI heart resulted in a decrease in the MI area and improvement in cardiac function after 8–12 weeks. The CMs, ECs, and SMCs derived from ieCPCs survived for a long time.
Conclusion: the next step in regenerative medicine is promising
The present study by Zhang et al. provides important insights into cardiac reprogramming, but also raises several interesting questions. A first question involves the molecular mechanism of maintaining ieCPCs in BACS medium and differentiation into all three lineages (CMs, ECs, and SMCs). In this study, transcriptome analysis demonstrated important similarities between ieCPCs and CPCs derived from mouse embryonic stem cells. Epigenetic and phosphoproteomic analyses are expected to answer the question more profoundly in the future. Second, how can we control the ratio of CMs, SMCs, and ECs that differentiate from ieCPCs in vivo? Transplantation of ieCPCs into the MI heart improves cardiac function, but which cells differentiate from ieCPCs in vivo is not clear. Thus, we should elucidate the interaction between differentiating cell and niche-derived signals that affect ieCPCs.
The heart is composed of various groups of cells, including blood vessel ECs, SMCs, nerve cells, and cardiac fibroblasts. Judging from the absolute number of cells comprising the heart, CMs only account for approximately 30% of heart cells, whereas cardiac fibroblasts constitute approximately 50% of this organ (15).
When a large number of CMs undergo necrosis following MI, the number of cardiac fibroblasts increases in the infarcted area. Heart rupture can be prevented by replacing the infarcted area with fibrous tissue; however, fibroblasts can result in low cardiac function and a potentially fatal arrhythmic focus.
Comparing ex vivo generation of CMs via differentiation of iPSCs versus direct reprogramming, the strategy using iPSCs is clearly far more advanced at this stage. Expandability and efficiency of cardiac induction are obviously major advantages of iPSCs over iCMs. However, direct reprogramming in vivo is associated with several theoretical advantages that may solve many of the challenges and issues associated with cell therapies (16).
The new strategy utilizing ieCPCs has new advantages of self-expandability and differentiation of the three cell types of the heart. We hope to utilize regenerative medicine-based therapies to treat patients with severe heart failure, potentially employing CMs derived from iPSCs, iCMs, and ieCPCs.
Acknowledgements
Funding: H Yamakawa’s work is supported by the Grant-in-Aid for Scientific Research (C) from the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76. [Crossref] [PubMed]
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861-72. [Crossref] [PubMed]
- Fujita J, Fukuda K. Future prospects for regenerated heart using induced pluripotent stem cells. J Pharmacol Sci 2014;125:1-5. [Crossref] [PubMed]
- Shimoji K, Yuasa S, Onizuka T, et al. G-CSF promotes the proliferation of developing cardiomyocytes in vivo and in derivation from ESCs and iPSCs. Cell Stem Cell 2010;6:227-37. [Crossref] [PubMed]
- Seki T, Yuasa S, Oda M, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell 2010;7:11-4. [Crossref] [PubMed]
- Hattori F, Chen H, Yamashita H, et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods 2010;7:61-6. [Crossref] [PubMed]
- Egashira T, Yuasa S, Suzuki T, et al. Disease characterization using LQTS-specific induced pluripotent stem cells. Cardiovasc Res 2012;95:419-29. [Crossref] [PubMed]
- Tohyama S, Hattori F, Sano M, et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2013;12:127-37. [Crossref] [PubMed]
- Tohyama S, Fujita J, Hishiki T, et al. Glutamine Oxidation Is Indispensable for Survival of Human Pluripotent Stem Cells. Cell Metab 2016;23:663-74. [Crossref] [PubMed]
- Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010;142:375-86. [Crossref] [PubMed]
- Efe JA, Hilcove S, Kim J, et al. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol 2011;13:215-22. [Crossref] [PubMed]
- Chong JJ, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014;510:273-7. [Crossref] [PubMed]
- Yamakawa H, Ieda M. Strategies for heart regeneration: approaches ranging from induced pluripotent stem cells to direct cardiac reprogramming. Int Heart J 2015;56:1-5. [Crossref] [PubMed]
- Zhang Y, Cao N, Huang Y, et al. Expandable Cardiovascular Progenitor Cells Reprogrammed from Fibroblasts. Cell Stem Cell 2016;18:368-81. [Crossref] [PubMed]
- Mewton N, Liu CY, Croisille P, et al. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 2011;57:891-903. [Crossref] [PubMed]
- Sadahiro T, Yamanaka S, Ieda M. Direct cardiac reprogramming: progress and challenges in basic biology and clinical applications. Circ Res 2015;116:1378-91. [Crossref] [PubMed]
Cite this article as: Yamakawa H, Fukuda K. Comment on: Expandable cardiovascular progenitor cells reprogrammed from fibroblasts. Stem Cell Investig 2016;3:89.


