Bone marrow derived stem cell therapy for type 2 diabetes mellitus
Introduction
Diabetes incidence is on the rise, afflicting 8–10% of the world population with as many suspected of having pre-diabetes. This incidence will likely double in the next few decades due to the widespread sedentary lifestyles and dietary indiscretions. Ninety-five percent of the patients inflicted with diabetes have type 2 diabetes (T2D) which carries a huge burden on the patient and society in terms of complications, psychological impact and economic cost (1-3).
T2D patients have impaired insulin production due to pancreatic beta cell degeneration and dysfunction associated with abnormal signaling downstream the pathway from the insulin receptor resulting in peripheral insulin resistance (4). There are also inflammatory, immune and microvascular abnormalities in T2D evident through cytokine disturbances resulting in clinical manifestations and widespread complications (4-7).
Newer categories of diabetic drugs are helping to delay the different complications; nevertheless, because of the progressive nature of the disease, there comes a point where the choices become limited as the injectable insulin becomes necessary (7,8).
Cellular therapies for diabetes
Progenitor stem cells have the capacity to self-renew and differentiate into different cell types in vitro depending on their micro-environment. There are several types of stem cells under investigation for the treatment of T2D. These include the bone marrow mononuclear stem cell (BM-MNSC), the peripheral hematopoietic stem cells, the mesenchymal stem cells (MSCs), the embryonic, the induced pluripotent stem cells, and others (9,10).
The BM-MNSCs contain several types of cells including the hematopoietic precursors, the MSCs and mature differentiated cells. When the marrow cells are infused peripherally, different mechanisms of action take place. The stem cells home not only to the bone marrow but also to injured areas where they are activated to secrete different cytokines and growth factors (11-13). The BM-MNSCs, probably by paracrine effects, stimulate angiogenesis and vascularization in ischemic areas (14,15). Furthermore, they probably stimulate the endogenous stem cells to proliferate and contribute to the repair process (16). The final result is repair, vascularization of under-perfused areas and modulation of the inflammatory and fibrosis processes.
Approaches like immune-suppressive therapies for type 1 diabetes including chemotherapeutic agents, antithymocyte globulins and similar agents followed sometimes by autologous bone marrow or peripheral stem cell transplantation has shown efficacy but at the expense of significant side effects (17-19). Other groups like Voltarelli et al. used autologous bone marrow transplantation following high-dose immuno-suppression in newly diagnosed type 1 DM in a prospective design on 15 patients. Fourteen patients became insulin-free for a median of 6 or more months with acceptable toxicity (20).
Different types of stem cells have been shown to differentiate in vitro into islet cells but the evidence for in vivo differentiation is not readily available at this point (21,22). In addition, several clinical trials have shown a significant benefit when using autologous BM-MNSCs for the patients with T2D. BM-MNSCs are good candidates for T2D patients’ treatment without immune-suppressive therapy for all the expected benefits listed above with the fact that their preparation and ex-vivo manipulation are simple and within reach at several tertiary care centers. No short or long term safety issues have been reported to hinder this approach (23-25).
Wang et al. reported using autologous bone marrow stem cells in combination with hyperbaric oxygen for the treatment of T2D. Thirty-one patients were given bone marrow derived stem cells into the major pancreatic arteries. The mean HbA1c decreased by more than 1.5% within 30 days after the treatment. At 3 months, the C-peptide increased reflecting improved insulin production. All patients had a significant reduction of their need for insulin and oral hypoglycemic drugs (26).
Wu et al. reported a single-center, randomized; trial randomly assigned 80 patients into four groups receiving BM-MNSCs with or without Hyperbaric therapy. At 12 months, the C-peptide of the patients receiving BM-MNC were significantly improved. The treatment was very well tolerated with minor adverse events including transient abdominal pain and local hemorrhage. The hyperbaric treatment did not seem to add any benefits to the BM-MNSCs (27).
Hu et al. reported treating one hundred and eighteen patients with T2D with BM-MNSCs or insulin intensification therapy based on the patient’s choice. The BM-MNSCs were injected into the patient’s pancreas via a catheter. No acute or chronic side effects were reported. The HbA1c and C-peptide were significantly better proving safety and efficacy of this approach compared to the control group (28).
Bhansali et al. reported on a prospective, randomized, placebo-controlled study using BMMNSCs in T2DM. Eleven patients received two doses of BM-MNSCs 12 months apart. Nine out of the 11 patients (82%) in the intervention group achieved more than 50% reduction in the insulin requirements. Ten out of 11 (91%) of the patients could maintain HbA1c <7% in the intervention group, whereas 6 out of 10 in the control group did (60%). The C-peptide significantly increased in the treated cases compared to controls (29).
Finally, a recent meta-analysis of fifteen trials concluded that both BM-MNSCs and peripheral blood mononuclear cells resulted in improvement of the HbA1C, fasting plasma glucose, C-peptide levels and endogenous insulin production at 12 months in the majority of treated patients (30).
Patients and methods
The study involved six patients aged 33 to 72, with proven, uncontrolled T2D. The patients had to have an elevated HbA1C over 7% for over a year, normal endogenous insulin C-peptide, and absence of anti-glutamic acid decarboxylase antibodies (Anti-GAD). The patients may have diabetic complications but had to have a stable, non-progressive course with stable cardiac, respiratory, renal and hepatic functions over the 3 months preceding the study. They had to have no active infections and be able and willing to consent to the study protocol.
Diet and exercise advice were offered to each patient with the objective of moderate reduction in caloric intake, and physical activity to optimize the conditions for success. Each patient’s bone marrow was collected under general anesthesia in a sterile environment. Approximately, 5 mL/kg of marrow were collected from the posterior iliac crests through eight puncture sites. Jamshidi bone marrow needle, gauge 8 was used to aspirate the marrow into a syringe with heparin sodium in a 10% solution. The BM-MNSCs were washed and separated using density gradient centrifugation then counted. The cells were subjected to microbiologic, morphologic, and serologic testing. The cells were then suspended in 50 mL of saline to be injected. The cells were kept at 4 °C overnight.
On the second day, the patients underwent infusion of the BM-MNSCs into the superior mesenteric and celiac arteries accessed via the femoral artery by an interventional radiologist. The volume was split equally between the two arteries. The patients were discharged 24 h after the procedure if they were hemodynamically stable with no bleeding. After completion, each patient was followed up by an endocrinologist on a monthly basis for the first 6 months then every 3 months thereafter. A form had to be completed each visit including glucose levels, need for diabetic medications, HbA1C and any change in the diabetic complications. Participants were also reminded each visit to adhere to the lifestyle changes.
Results
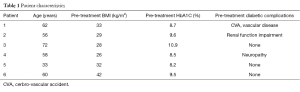
Six patients were enrolled between 2010 and 2013 on this study. The ages varied between 33 and 72. All patients had a HbA1C over 7.0% for the year preceding enrolment with stable renal, hepatic, and cardiopulmonary status. The patients received autologous BM-MNSCs as described above. Three patients had diagnosed diabetic complications including one with renal impairment, one with thromboembolic and small vessel disease and one with bilateral symmetric peripheral neuropathy of the lower limbs. Table 1 summarizes the patient characteristics illustrating that most patients have a BMI classified as overweight or obese with poor control of their T2D based on their HbA1C (Table 1).
Full table
Five of six patients normalized their HbA1C within 12 weeks of administration to less than 7%. The patient with peripheral neuropathy reported gradual symptomatic improvement over 10 months after the infusion while the patient with renal function impairment showed stabilization of his renal function.
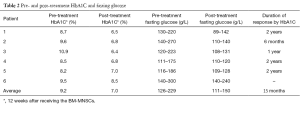
The duration of this response as measured by normal HbA1C, varied significantly between 6 and 24 months with an average of 15 months. Weight loss during the therapeutic process was not a significant contributor to the results. The pretreatment body mass index (BMI) ranged between 26 and 42.
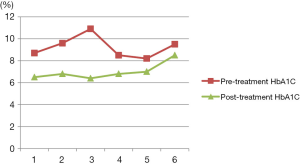
The pre-treatment HbA1C ranged between 8.2% to 10.9%. The C-peptide was not obtained at regular intervals to assess the endogenous insulin production. This was reflected by the fasting glucose levels which ranged between 116 and 270 before the treatment and 89 and 142, 3 months later. The fasting glucose levels, response by HbA1C and the duration of the response are detailed in Table 2 and Figure 1.
Full table
While the HbA1C ranged between 8.2% and 10.9% before the treatment, the levels dropped to 6.4% and 8.5% in 3 months after therapy. The HbA1C levels were over 7% only in the non-responding patient (Table 2).
On the average, the pretreatment HbA1C dropped from 9.2% before the treatment to 7% after it. This was a 2.2-point drop or about 24% improvement. See Figure 1.
There was also a parallel reduction of the need for both insulin and oral medications. The general subjective report of the patients was good feelings of health improvement, more energy and positive quality of life (Figure 1).
Discussion
Diabetes mellitus remains a major health problem that afflicts a growing population of sedentary, obese and digitally avid population shifting away from healthy lifestyles. The endemic levels it reached are due to several factors and their complications may involve several organs and affect the well being of the patients significantly. There is no question that cellular therapy is one of the favored future methods being investigated not only to treat but hopefully to cure T2D (23-25).
Giving BM-MNSCs is not a new idea. As discussed earlier, several groups have shown that we can use the BM-MNSCs to stop or taper the manifestations, complications and possibly halt T2D for a variable period of time (26-30).
Our patients had a 24% reduction of their HbA1C on the average, reduction of their fasting glucose and the medications needed as well as subjective improvement of their health perception. Unfortunately, the C-peptide was not done regularly to report the change of the endogenous insulin production. Our results are similar in general to what was reported by other groups using the BM-MNSCs as well as other types of stem cells including the MSCs.
Some of the reported methods require a complicated preparation and would be difficult to apply in the daily practice. For example, Ramiya et al. isolated pancreatic ductal epithelial cells from diabetic mice in cultures, and induced them to produce functioning islets in vitro before re-implantation to reverse the insulin-dependence (31).
Soria et al. used embryonic stem (ES) cells to transform it into insulin-secreting cell clones offering an alternative to gene therapy (32). Many studies showed the usefulness of this approach in animal models but the applicability in patient care and costs are not clear yet but are likely to make those treatments out of reach for most patients.
More practical approaches but still very expensive, are the allogeneic MSCs readily available from some burgeoning companies. Skyler et al. published a milestone phase III, placebo controlled, multicenter clinical study recently using allogeneic MSCs at an escalating dose. At week 12, the target HbA1C of 7 mg/dL or less was achieved MSCs by 33% (5 of 15) of the subjects who received the 2.0×106/kg dose and 50% of those receiving 1.0×106/kg. The source of the MSCs was adult allogeneic bone marrow MSCs precursor cells. Only one dose of MSCs was given intravenously with no significant side effects (33).
In addition to their efficacy in controlling the HbA1C and glucose levels, evidence also suggests that patients with diabetic complications like critical limb ischemia with extensive ulceration and gangrene may benefit from injection of autologous BM-MNSCs. Six patients received intralesional BM-MNSCs at various sites of the ulcer and its surroundings after debridement showed significant improvement at 6-month follow-up. The lower limb pain, mean toe-brachial index, limb perfusion and ulcers improved significantly. The procedure was safe with no adverse outcomes (34).
Although there is generally little or no clear evidence of in-vivo BM-MNSCs differentiation into pancreatic beta cells (35,36), there are other potential mechanisms to explain this improvement including the neo-vascularization, endothelial repair, inflammatory environment modulation and endogenous stem cell stimulation through paracrine mediators (12-16).
There is also evidence that the BM-MNSCs may work in type 1 diabetes, the insulin dependent type (37). The mechanism is probably similar to what was discussed but with possible larger immune modification role.
The idea of giving bone marrow cells seems to work and achieve similar results to what was reported by other groups (26-30). The important part is that this could be done in a simple set up where the patient can be hospitalized for a single night and discharged the second day to be followed up and monitored. There is no theoretical reason not to repeat this procedure if necessary when the diabetes control is lost. Needless to say, these results need to be validated and further expanded in a large scale randomized study.
Conclusions
Our pilot study adds to the growing body of evidence that stem cell therapy, whether BM-MNSCs or MSCs or embryonic cells have a role in controlling T2D in most patients but the best cell type, dose and administration schedule are yet to be determined. Should it be every 6 months or as soon as the patient shows signs of recurrence? Should it be combined with other treatments? Should the cells be given locally or intravenously systemically? Should growth factors be used along with the stem cells?
We believe the BM-MNSCs are the easiest to prepare and use among the available stem cell types, cheaper in general and more readily available to most tertiary care centers. The methods used have been shown here and in several other studies to be safe and easy to tolerable, requiring only 24–36 h of hospitalization. We believe those methods are probably the most suitable for larger, well controlled, randomized studies as the field advances into more acceptable standards. A lot of caution has to be exerted to expand a parallel regulatory work to channel these efforts into the correct scientific methods. It is clear that it will be a long bumpy road before those therapies become mainstream treatments.
Acknowledgements
The authors acknowledge the contribution of the patients and their families to this work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: All patients involved consented to the publication of their data.
References
- Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047-53. [Crossref] [PubMed]
- Cefalu WT, Tamborlane WV, Skyler JS. Type 1 diabetes at a crossroads! Diabetes Care 2015;38:968-70. [Crossref] [PubMed]
- Available online: Http://www.diabetes.org/diabetes-basics/statistics/. Accessed December 9, 2015.
- Cersosimo E, Triplitt C, Mandarino LJ, et al. Pathogenesis of Type 2 Diabetes Mellitus. In: De Groot LJ, Chrousos G, Dungan K, et al. editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-2015. Last Update: May 28, 2015.
- Butler AE, Janson J, Bonner-Weir S, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102-10. [Crossref] [PubMed]
- Folli F, Okada T, Perego C, et al. Altered insulin receptor signalling and β-cell cycle dynamics in type 2 diabetes mellitus. PLoS One 2011;6:e28050. [Crossref] [PubMed]
- Vijan S. In the clinic. Type 2 diabetes. Ann Intern Med 2015;162:ITC1-16. [Crossref] [PubMed]
- Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006;368:1681-8. [Crossref] [PubMed]
- Shapiro AM. Islet transplantation in type 1 diabetes: ongoing challenges, refined procedures, and long-term outcome. Rev Diabet Stud 2012;9:385-406. [Crossref] [PubMed]
- Rezania A, Bruin JE, Riedel MJ, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 2012;61:2016-29. [Crossref] [PubMed]
- Shyu WC, Lee YJ, Liu DD, et al. Homing genes, cell therapy and stroke. Front Biosci 2006;11:899-907. [Crossref] [PubMed]
- Kim H, Park JS, Choi YJ, et al. Bone marrow mononuclear cells have neurovascular tropism and improve diabetic neuropathy. Stem Cells 2009;27:1686-96. [Crossref] [PubMed]
- Duong Van Huyen JP, Smadja DM, Bruneval P, et al. Bone marrow-derived mononuclear cell therapy induces distal angiogenesis after local injection in critical leg ischemia. Mod Pathol 2008;21:837-46. [Crossref] [PubMed]
- Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369-77. [Crossref] [PubMed]
- Tse HF, Kwong YL, Chan JK, et al. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet 2003;361:47-9. [Crossref] [PubMed]
- Nakano-Doi A, Nakagomi T, Fujikawa M, et al. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem Cells 2010;28:1292-302. [PubMed]
- Oh SH, Muzzonigro TM, Bae SH, et al. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest 2004;84:607-17. [Crossref] [PubMed]
- Amin AH, Abd Elmageed ZY, Nair D, et al. Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice. Lab Invest 2010;90:985-96. [Crossref] [PubMed]
- Domínguez-Bendala J, Inverardi L, Ricordi C. Stem cell-derived islet cells for transplantation. Curr Opin Organ Transplant 2011;16:76-82. [Crossref] [PubMed]
- Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2007;297:1568-76. [Crossref] [PubMed]
- Hess D, Li L, Martin M, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol 2003;21:763-70. [Crossref] [PubMed]
- Kahan BW, Jacobson LM, Hullett DA, et al. Pancreatic precursors and differentiated islet cell types from murine embryonic stem cells: an in vitro model to study islet differentiation. Diabetes 2003;52:2016-24. [Crossref] [PubMed]
- Dave SD, Vanikar AV, Trivedi HL. Ex vivo generation of glucose sensitive insulin secreting mesenchymal stem cells derived from human adipose tissue. Indian J Endocrinol Metab 2012;16 Suppl 1:S65-9. [Crossref] [PubMed]
- Bernardi S, Severini GM, Zauli G, et al. Cell-based therapies for diabetic complications. Exp Diabetes Res 2012;2012:872504.
- Liew CG, Andrews PW. Stem cell therapy to treat diabetes mellitus. Rev Diabet Stud 2008;5:203-19. [Crossref] [PubMed]
- Wang L, Zhao S, Mao H, et al. Autologous bone marrow stem cell transplantation for the treatment of type 2 diabetes mellitus. Chin Med J (Engl) 2011;124:3622-8. [PubMed]
- Wu Z, Cai J, Chen J, et al. Autologous bone marrow mononuclear cell infusion and hyperbaric oxygen therapy in type 2 diabetes mellitus: an open-label, randomized controlled clinical trial. Cytotherapy 2014;16:258-65. [Crossref] [PubMed]
- Hu J, Li C, Wang L, et al. Long term effects of the implantation of autologous bone marrow mononuclear cells for type 2 diabetes mellitus. Endocr J 2012;59:1031-9. [Crossref] [PubMed]
- Bhansali A, Asokumar P, Walia R, et al. Efficacy and safety of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus: a randomized placebo-controlled study. Cell Transplant 2014;23:1075-85. [Crossref] [PubMed]
- Wang ZX, Cao JX, Li D, et al. Clinical efficacy of autologous stem cell transplantation for the treatment of patients with type 2 diabetes mellitus: a meta-analysis. Cytotherapy 2015;17:956-68. [Crossref] [PubMed]
- Ramiya VK, Maraist M, Arfors KE, et al. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med 2000;6:278-82. [Crossref] [PubMed]
- Soria B, Skoudy A, Martín F. From stem cells to beta cells: new strategies in cell therapy of diabetes mellitus. Diabetologia 2001;44:407-15. [Crossref] [PubMed]
- Skyler JS, Fonseca VA, Segal KR, et al. Allogeneic Mesenchymal Precursor Cells in Type 2 Diabetes: A Randomized, Placebo-Controlled, Dose-Escalation Safety and Tolerability Pilot Study. Diabetes Care 2015;38:1742-9. [Crossref] [PubMed]
- Subrammaniyan R, Amalorpavanathan J, Shankar R, et al. Application of autologous bone marrow mononuclear cells in six patients with advanced chronic critical limb ischemia as a result of diabetes: our experience. Cytotherapy 2011;13:993-9. [Crossref] [PubMed]
- Choi JB, Uchino H, Azuma K, et al. Little evidence of transdifferentiation of bone marrow-derived cells into pancreatic beta cells. Diabetologia 2003;46:1366-74. [Crossref] [PubMed]
- Lechner A, Yang YG, Blacken RA, et al. No evidence for significant transdifferentiation of bone marrow into pancreatic beta-cells in vivo. Diabetes 2004;53:616-23. [Crossref] [PubMed]
- Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2009;301:1573-9. [Crossref] [PubMed]
Cite this article as: Wehbe T, Chahine NA, Sissi S, Abou-Joaude I, Chalhoub L. Bone marrow derived stem cell therapy for type 2 diabetes mellitus. Stem Cell Investig 2016;3:87.