p38α-mediated purine metabolism is linked to exit from quiescence of hematopoietic stem cells
Hematopoietic stem cells (HSCs) are atop of a hierarchy of the hematopoietic and immune system, continuously replenishing short-lived mature blood and immune cells. Like other adult stem cells, HSCs have two key characteristics—self-renewal and the capacity to produce lineage-committed progenies (1). HSCs also adopt strategies throughout life to protect themselves from a variety of exogenic or endogenic threats to prevent their exhaustion (2). Tracer experiments show that there are stratified classes of HSCs based on their potential to reconstitute the hematopoietic system of a recipient (3). Dormant or quiescent HSCs are at the top of the HSC subpopulations and reside in endosteal niches in bone marrow cavities which provide a microenvironment suitable to keep them in a quiescent G0 stage of the cell cycle, a pivotal property of stem cells required to maintain their genomic integrity by minimizing DNA damage, cellular respiration, and cell division (1-4). However, for homeostatic hematopoiesis, some HSCs retain a certain degree of proliferative capacity for self-renewal and generation of multipotent progenitors (2). This task is performed by homeostatic HSCs. These cells attain characteristic phenotypes of dormant HSCs in cases that they are exhausted (3). It seems that HSCs with more proliferative history progressively lose their self-renewal capacity when transplanted into irradiated recipients (3).
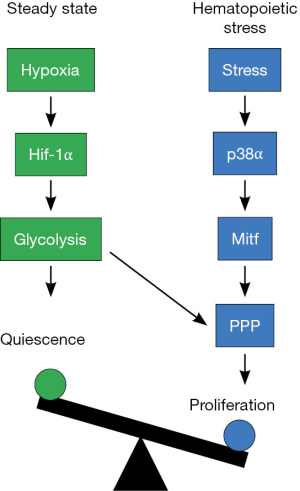
Hypoxic conditions of HSC niches are critical in maintaining the quiescent features of HSCs (2). Cellular responses to hypoxia are mediated by hypoxia-inducible factors (Hifs). Among them, Hif-1α is known to mediate HSC dormancy by activating transcription of genes controlling glycolysis (5-8). As a consequence, HSCs use anaerobic respiration instead of mitochondrial oxidative phosphorylation as the main source of energy. Slow metabolic rates thus may provide an explanation for one mechanism through which Hif-1α promotes HSC quiescence. However, Hif-1α controls transcription of numerous genes involved in cellular processes, including the cell cycle, survival, and autophagy (9). In addition, enzymes regulating energy metabolism and their metabolites may play an indispensable role in other than metabolic functions for maintenance of HSC characteristics (10).
Magnitudes of hematopoiesis are determined by the repopulation demand of the blood system. For example, active hematopoiesis occurs during hematopoietic stress, including infection, irradiation, treatment with chemotherapeutics agents, or transplantation of bone marrow cells (2). Infections with bacterial or fungal pathogens require an explosive number of phagocytes to clear the invasive microbes. In this case, emergency myelopoiesis is stimulated mainly by pathogen-associated molecular patterns (DAMPs) and cytokines and a large number of myeloid cells infiltrate to infection sites and mediates sequential inflammatory events linked to pathogen clearance (11). Genotoxic insults such as ionizing irradiation and toxic chemotherapeutics instigate active hematopoiesis to refill a massive loss of adult blood cells. In either situation, quiescent HSCs are sensitized to enter into the cell cycle. IFN-α and G-CSF are well known cytokines to activate this process during hematopoietic stress (2). However, cell intrinsic factors controlling the switching mechanisms of HSCs from dormancy to self-renewal remains largely unknown.
Despite its low inefficiency in generating cellular ATP, the glycolytic pathway provides key metabolic intermediates linked to the biosynthesis of ribose for nucleotide (glucose-6-phosphate into pentose phosphatase), amino acids (3-phosphoglycerate into the serine biosynthetic pathway) and fatty acids (pyruvate into the TCA cycle for citrate) (10). In addition, glycolytic metabolism allows for the generation of NADH which is used by numerous enzymes as s cofactor. During hematopoietic stress, HSCs exit from quiescence but maintain the glycolytic pathway. In this respect, the PI3K-MAPK-mTOR signaling pathway may be critical in connecting between increased glycolysis and activation of the cell cycle and subsequently increasing proliferation of HSCs. Indeed, Ito and colleagues (12) found that oxidative stress activates p38α MAPK which is known to govern stress responses (13). They demonstrated that treatment with a p38α MAPK inhibitor during transplantation restores HSC function and number and consequently preventing HSC aging.
Recent studies by Karigane and colleagues significantly advance our understanding of how p38α MAPK promotes hematopoietic recovery during hematopoietic stress (14). They used an inducible conditional approach to assess p38α MAPK function in HSCs. Deletion of p38α in young mice had no effect on steady-state hematopoiesis but in hematopoietic stress situations, HSCs lacking p38α exhibited defective hematopoiesis. The impaired hematopoiesis of p38α-depleted HSCs seems to be intrinsic, because they displayed no abnormality in homing, apoptosis, or ROS production and p38α deficiency in HSC niches has no influence on hematopoiesis during hematopoietic stress. As cell cycle progression in HSCs is initiated during hematopoietic stress, they compared the proliferative ability of wild-type and p38α-deficient HSCs in transplantation settings, finding that transplanted long-term (LT)-HSCs of p38α-deficient mice had impaired proliferation in recipients. Gene expression profiling also confirmed that there was lower expression of genes related to HSC markers and proliferation in transplanted p38α-deficient LT-HSCs. Analysis of metabolome also showed that levels of most metabolites in LKS cells of wild-type mice were increased after bone marrow transplantation compared to LKS cells at steady state but a majority of the upregulated metabolites was reduced in p38α-deficient LKS cells after transplantation. However, Karigane and colleagues noticed accumulation of glycine and aspartic acid, two amino acids used for purine metabolism, and reduction of allantoin, a final production of purine catabolism, in LSK cells lacking p38α after transplantation. Consistently, expression of inosine-5’-monophosphate dehydrogenase 2 (Impdh2), the rate-limiting enzyme of guanosine monophosphate (GMP) was shown to be decreased in p38α-deficient LT-HSCs early after bone marrow transplantation. The authors further demonstrated that Mitf (microphthalmia-associated transcription factor) is a critical downstream transcription factor of p38α MAPK signaling regulating expression of Impdh2. Importantly, overexpression of Impdh2 in LKS cells of p38α- or Mitf-deficient mice rescues the impairment of their short- and long-term repopulation capacity.
In summary, Karigane and colleagues identify p38α MAPK signaling as central checkpoint regulating exit from HSC quiescence during hematopoietic stress. As p38α MAPK signaling also negatively regulates HSC quiescence at steady state and consequently, reduces their lifespan, steady state and hematopoietic stress are likely to induce a common signaling point upstream of p38α MAPK in HSCs. It will be interesting to elucidate the upstream signaling point linked to HSC proliferation. Another important issue is to reveal how a crosstalk between HIf-1α and p38α regulating expression of glycolysis-related genes or pentose phosphate pathway-related genes, respectively, occurs in HSCs during hematopoietic stress (Figure 1). Nonetheless, the findings of Karigane and colleagues provide an important insight into a mechanism of hematopoiesis during hematopoietic stress and may also open up novel therapeutic opportunities via the p38α-Mitf-purine metabolism pathway for hematopoietic stem and progenitor-related leukemia.
Acknowledgements
None.
Footnote
Provenance: This is an invited Editorial commissioned by Editor-in-Chief Zhizhuang Joe Zhao (Pathology Graduate Program, University of Oklahoma Health Sciences Center, Oklahoma City, USA).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 2008;132:631-44. [Crossref] [PubMed]
- Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nat Rev Immunol 2010;10:201-9. [Crossref] [PubMed]
- Säwén P, Lang S, Mandal P, et al. Mitotic History Reveals Distinct Stem Cell Populations and Their Contributions to Hematopoiesis. Cell Rep 2016;14:2809-18. [Crossref] [PubMed]
- Blanpain C, Mohrin M, Sotiropoulou PA, et al. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell 2011;8:16-29. [Crossref] [PubMed]
- Simsek T, Kocabas F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 2010;7:380-90. [Crossref] [PubMed]
- Takubo K, Goda N, Yamada W, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 2010;7:391-402. [Crossref] [PubMed]
- Takubo K, Nagamatsu G, Kobayashi CI, et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell 2013;12:49-61. [Crossref] [PubMed]
- Wang YH, Israelsen WJ, Lee D, et al. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell 2014;158:1309-23. [Crossref] [PubMed]
- Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008;30:393-402. [Crossref] [PubMed]
- O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol 2016;16:553-65. [Crossref] [PubMed]
- Boettcher S, Manz MG. Sensing and translation of pathogen signals into demand-adapted myelopoiesis. Curr Opin Hematol 2016;23:5-10. [Crossref] [PubMed]
- Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 2006;12:446-51. [Crossref] [PubMed]
- Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 2013;13:679-92. [Crossref] [PubMed]
- Karigane D, Kobayashi H, Morikawa T, et al. p38α Activates Purine Metabolism to Initiate Hematopoietic Stem/Progenitor Cell Cycling in Response to Stress. Cell Stem Cell 2016;19:192-204. [Crossref] [PubMed]
Cite this article as: Kwon B. p38α-mediated purine metabolism is linked to exit from quiescence of hematopoietic stem cells. Stem Cell Investig 2016;3:69.


