TET2 mutations were predictive of inferior prognosis in the presence of ASXL1 mutations in patients with chronic myelomonocytic leukemia
Introduction
Somatic mutations have been detected in about 90% of patients with chronic myelomonocytic leukemia (CMML) and the most frequent mutations occur in epigenetic regulators (TET2, 40–60%), histone modification and chromatin regulation (ASXL1, 30–50%), splicing components (SRSF2, 30–50%), transcription factors (RUNX1, 10–20%), and signaling regulator genes (SETBP1, 10–20%) (1-5). In proximately, over 40% CMML patients have at least two mutations (3,5,6). The diverse combinations of mutations detected in CMML suggest multi-step pathogenesis of the disease in some cases. For example, ASXL1 and TET2 mutations are the most frequent mutations and may be independent drivers of CMML in some patients (7), and the combination of TET2/SRSF2 mutations and ASXL1/SETBP1 mutations are consistent with a two-step ‘linear’ model of CMML development (8). In vitro studies have shown that ASXL1 mutations enhance the de-ubiquitinase activity of the ASXL1-BAP1 (BRCA associated protein 1) complex, which then cooperates with loss of TET2 to skew towards myeloid development (9). In a recent study, Patnaik et al. (6) demonstrated prognostic interaction of ASXL1 and TET2 mutations, which showed that TET2 mutations predict favorable survival in the absence of ASXL1 mutations. In this study, ASXL1 and TET2 mutations were detected in 141 patients with CMML and the interaction of ASXL1 and TET2 mutations in the prognostication of CMML were conducted.
Patients and methods
From July 2007 to July 2015, 141 adult patients were reviewed and diagnosed as CMML according to the 2008 World Health Organization criteria (10). All patients received symptomatic treatment (blood transfusions, hydroxyurea, etc.). Risk stratifications of patients was based on Mayo Prognostic Model and Mayo Molecular Model (4,11). Overall survival (OS) was calculated from the date of first referral to the date of death (uncensored) or last contact (censored). This study was approved by the Ethical Committees of the Institute of Hematology, Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC) following principles of the Declaration of Helsinki and all patients have signed informed consent.
Genomic DNA of bone marrow mononuclear cells collected at diagnosis was extracted using conventional methods. Mutation analysis was performed according to previously published methods (5). Sequencing was bi-directional. Sequence traces were analyzed with Mutation Surveyor Software (Applied Biosystems Genetic Analyzers). Single nucleotide polymorphisms previously annotated (www.hapmap.org) were discarded. Frameshift, nonsense and missense of TET2 mutations were considered pathogenic while only frameshift and nonsense of ASXL1 mutations were considered as pathogenic (only frameshift and nonsense mutations of ASXL1 independently impacting OS in previously published research).
Numerical variables are presented as medians and ranges. Categorical variables are described as counts and relative frequencies (%). Comparisons between categorical variables were performed using χ2 tests. Comparisons between continuous variables were performed using the Kruskal-Wallis U-test. Survival was analyzed by the Kaplan-Meier method and compared using the log-rank test. A Cox model was used to identify the prognostic variables. All the statistical analyses were conducted with SPSS version 18.0. All the P values are two-tailed, and statistical significance was set at P<0.05.
Results
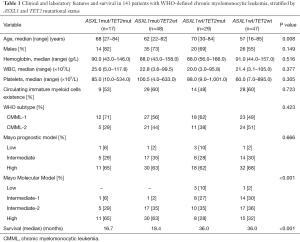
The median age was 63 (range, 16–85) years, with a male 95 (67%) predilection. Following the WHO criteria, 80 (57%) of patients were identified as CMML-1 and the remainder as CMML-2, with median OS of 21 and 18 months, respectively. With a median follow-up of 15.4 months, 80 (57%) deaths were documented. Sixty-five (46.1%) of the CMML patients harbored ASXL1 mutations (frameshift and nonsense), and 46 (32.6%) had a TET2 mutation (frame shift, nonsense and missense). Following the Mayo Prognostic Model, 64.5% were classified as high, 31.2% as intermediate and 4.3% as low risk, while according to the Molecular Mayo Model, 45.4% corresponded to high, 34.8% to intermediate-2, 17.0% to intermediate-1 and 2.8% to low risk.
In univariate analysis, hemoglobin <100 g/L (P=0.004), presence of circulating immature myeloid cells (IMCs) (P=0.006), platelet <50×109/L (P=0.022) and presence of ASXL1 mutations (P<0.001) predict inferior prognosis while the presence of TET2 mutations not (P=0.922). In multivariable analysis, hemoglobin <100 g/L (P=0.008), presence of IMCs (P=0.015) and ASXL1 (P=0.001) mutations remained significant.
We also stratified these 141 patients into four mutational categories to find out prognostic interaction between ASXL1 and TET2 mutations: ASXL1mut/TET2mut (n=17), ASXL1mut/TET2wt (n=48), ASXL1wt/TET2mut (n=29) and ASXL1wt/TET2wt (n=47). There was no statistical significance of hemoglobin, WBC, platelets, existence of IMCs except the ages among the four groups (details shown in Table 1). OS of the four groups from inferior to favorable was ASXL1mut/TET2mut (16.7 months), ASXL1mut/TET2wt (18.4 months) and ASXL1wt/TET2mut (36.0 months) ASXL1wt/TET2wt (36.0 months) (Figure 1). We did confirm the significant favorable impact of TET2 mutations in the absence of ASXL1 mutations (P=0.580).
Full table
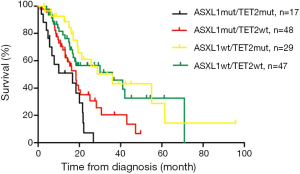
In multivariable analysis that included the Mayo Prognostic Model as a single variable along with ASXL1wt/TET2wt, the respective hazard ratios of ASXL1mut/TET2mut, ASXL1mut/TET2wt and ASXL1wt/TET2mut were 4.7 (95% CI, 2.2–10.3; P<0.001), 2.2 (95% CI, 1.1–4.2; P=0.025) and 1.3 (95% CI, 0.6–2.5; P=0.521).
Discussion
About 90% of patients with CMML had at least one somatic mutation, and over 20 relative genes were involved (6). The various combinations of genetic abnormalities in CMML did not only indicate a multi-step pathogenesis, but also likely contributed to the marked clinical heterogeneity of these disorders. In this study, we confirmed the negative prognostic impact on OS imparted by ASXL1 mutations, and also suggested an unfavorable prognostic impact from TET2 mutations in the presence of ASXL1 mutations. ASXL1 and TET2 mutations were considered as independent drivers of CMML (7), and ASXL1mut/TET2mut suggested two ‘lineage’ clone development and might lead to poorer prognosis than other subgroups in patients receiving symptomatic treatment.
Although ASXL1 mutations negatively impact survival, there is not efficient evidence of correlation between overall response rate to hypomethylating agents (HMAs) and ASXL1 mutations (12). Total 5-methyl-cytosine levels in TET2mut cases were significantly higher than TET2wt cases in CMML (13). Patnaik et al. (6) demonstrated TET2 mutations predict favorable survival in the absence of ASXL1 mutations. Bejar et al. (14) also reported higher abundance TET2 mutations were associated with better response to HMAs in the absence of ASXL1 mutations in myelodysplastic syndrome patients. Thus, we speculated that TET2 mutations may make up inferior OS imparted by ASXL1 mutations and lead to better OS in ASXL1wt cases under treatment with HMAs in other studies, but could not show any advantage with symptomatic treatment in our study. At the same time, the combined mutation of TET2 and ASXL1 were considered as complicated clonal combination of CMML, and thus ASXL1mut/TET2mut indicated an aggressive disease evolution.
Conclusions
In summary, our study showed ASXL1 mutations predict inferior OS, and additional TET2 mutations present with an aggressive disease evolution in the presence of ASXL1 mutations in CMML patients received symptomatic treatment.
Acknowledgements
Funding: This work supported by National Natural Science Funds (grant numbers 81470297, 81270585, 81370611, 81470295), Tianjin Key Natural Science Funds (grant number 12JCZDJC23900), National Public Health Grand Research Foundation (grant number 201202017), and National Key Technology R&D Program (grant number 2014BAI09B13).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Patnaik MM, Parikh SA, Hanson CA, et al. Chronic myelomonocytic leukaemia: a concise clinical and pathophysiological review. Br J Haematol 2014;165:273-86. [Crossref] [PubMed]
- Itzykson R, Kosmider O, Renneville A, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol 2013;31:2428-36. [Crossref] [PubMed]
- Padron E, Garcia-Manero G, Patnaik MM, et al. An international data set for CMML validates prognostic scoring systems and demonstrates a need for novel prognostication strategies. Blood Cancer J 2015;5:e333. [Crossref] [PubMed]
- Patnaik MM, Itzykson R, Lasho TL, et al. ASXL1 and SETBP1 mutations and their prognostic contribution in chronic myelomonocytic leukemia: a two-center study of 466 patients. Leukemia 2014;28:2206-12. [Crossref] [PubMed]
- Cui Y, Tong H, Du X, et al. Impact of TET2, SRSF2, ASXL1 and SETBP1 mutations on survival of patients with chronic myelomonocytic leukemia. Exp Hematol Oncol 2015;4:14. [Crossref] [PubMed]
- Patnaik MM, Lasho TL, Vijayvargiya P, et al. Prognostic interaction between ASXL1 and TET2 mutations in chronic myelomonocytic leukemia. Blood Cancer J 2016;6:e385. [Crossref] [PubMed]
- Itzykson R, Kosmider O, Renneville A, et al. Clonal architecture of chronic myelomonocytic leukemias. Blood 2013;121:2186-98. [Crossref] [PubMed]
- Itzykson R, Solary E. An evolutionary perspective on chronic myelomonocytic leukemia. Leukemia 2013;27:1441-50. [Crossref] [PubMed]
- Balasubramani A, Larjo A, Bassein JA, et al. Cancer-associated ASXL1 mutations may act as gain-of-function mutations of the ASXL1-BAP1 complex. Nat Commun 2015;6:7307. [Crossref] [PubMed]
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009;114:937-51. [Crossref] [PubMed]
- Patnaik MM, Padron E, LaBorde RR, et al. Mayo prognostic model for WHO-defined chronic myelomonocytic leukemia: ASXL1 and spliceosome component mutations and outcomes. Leukemia 2013;27:1504-10. [Crossref] [PubMed]
- Patnaik MM, Wassie EA, Padron E, et al. Chronic myelomonocytic leukemia in younger patients: molecular and cytogenetic predictors of survival and treatment outcome. Blood Cancer J 2015;5:e280. [Crossref] [PubMed]
- Yamazaki J, Taby R, Vasanthakumar A, et al. Effects of TET2 mutations on DNA methylation in chronic myelomonocytic leukemia. Epigenetics 2012;7:201-7. [Crossref] [PubMed]
- Bejar R, Lord A, Stevenson K, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood 2014;124:2705-12. [Crossref] [PubMed]
Cite this article as: Cui Y, Tong H, Du X, Li B, Gale RP, Qin T, Liu J, Xu Z, Zhang Y, Huang G, Jin J, Fang L, Zhang H, Pan L, Hu N, Qu S, Xiao Z. TET2 mutations were predictive of inferior prognosis in the presence of ASXL1 mutations in patients with chronic myelomonocytic leukemia. Stem Cell Investig 2016;3:50.


