Mesenchymal stem cell therapy for refractory scleroderma: a report of 2 cases
Introduction
Scleroderma is defined as an auto-immune condition characterized by the presence of skin thickening proximal to the metacarpophalangeal joints, with involvement of the fingers, fingertips. Scleroderma may also cause telangiectasia, abnormal nailfold capillaries, interstitial lung disease, pulmonary arterial hypertension, and Raynaud’s phenomenon.
The condition usually affects young women and may cause severe disability and suffering (1,2). The clinical picture is heterogeneous with abnormal deposition of collagen in the skin and other organs leading to multiple organ dysfunction. In many cases, it behaves as a progressive disease that may be life threatening. Antibodies to nuclear antibodies and inflammatory lymphocytic infiltration are early findings that develop in this disease (3,4). It is unclear how the vascular changes, the antibodies, and fibrosis are connected. The fibrosis unfortunately is irreversible. Significant mortality secondary to pulmonary, cardiac, renal, or gastrointestinal disease culminate the disability. The skin fibrosis can be severe enough to affect mobility and ambulation. The mortality at 5 years is about 30–58%, depending on the clinical features (5,6).
The mesenchymal stem cells (MSCs) have been shown to portray immune regulatory properties that make them good potential agents to treat autoimmune conditions. MSCs express low levels of the major histocompatibility complex I, and do not express MHC II or co-stimulatory molecules like B7-1, B7-2, or CD40 (7,8). MSC have been shown to be immunosuppressive through several mechanisms including inhibition of the immune T-cells and antigen presenting cells and through paracrine mechanisms allowing them to reset the immune microenvironment (9,10). In reality the MSCs expanded in culture from different sources are a heterogeneous mixture of cells (11,12). MSCs likely exert most of their effects by paracrine effects through a number of cytokines and growth factors packaged into their micro-vesicles. This results in inhibition of the B- and T-cells, monocyte maturation along with other well documented effects (13,14). The safety of the allogeneic MSCs have been clearly established through several published clinical trials given intravenously, intrathecally, intracisternally, or locally depending on the disease being treated (15,16). Allo-MSCs have been granted approval in Canada and New Zealand for the treatment of steroid refractory Graft vs. Host disease in children (Prochymal), as well as in South Korea (under the name Hearticellgram-AMI) (17,18).
Rituximab, on the other hand, have been shown also to result in good responses when compared with matched controls in diseases like scleroderma. Evidence shows that it may induce improved skin manifestations and prevention of further decline of the Forced Vital Capacity in affected patients with a good safety profile (19).
Patients and methods
Both patients had their disease confirmed by at least two rheumatologists. The procedures were fully explained and approved by the hospital ethical committee. All patients signed written informed consent. Umbilical cords (UCs) were used as a source of the allogenic MSCs. The UCs were processed within 2 h of delivery. The cords were disinfected with 70% alcohol then washed thoroughly with saline then digested mechanically and enzymatically with collagenase I under sterile conditions. The digested material was harvested and suspended in the growth medium. The pellets were counted using Trypan Blue stain after 16 and 40 h. The digested tissues were placed in 6-well plates and cultured in alpha modified Eagle’s medium (α-MEM) (Sigma, Germany), supplemented with 10% (v/v) fetal bovine serum (FBS) (Sigma, Germany), 2 mM L-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin and 1 µg/mL amphoterin B (Lonza, Belgium). The culture plate was placed in an incubator with saturated humidity at 5% CO2, at 37 °C.
The medium used was changed twice weekly once 80% confluence was reached. The cells were detached with trypsin, counted, and seeded in vented polystyrene tissue culture flask (Corning, USA) until passage 4. Finally, the cells were detached, counted, and washed then re-suspended in the infusion solution (20).
The 2 patients underwent 4 sessions of plasmapheresis first, followed by 1 g of rituximab then stem cell infusion intravenously. The MSCs were used at 2 million cells/kg, infused intravenously. The clinical assessment tool of the European Scleroderma Study Group (EsSG) activity index was used to assess the patients before and after treatment. The 2 patients were aged 28 and 30 years respectively. Both patients had received the recommended standards of care before referral. Patients’ characteristics are detailed in Table 1.
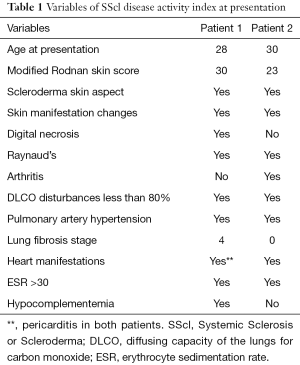
Full table
Results
Both patients experienced normalization of their pulmonary pressures as assessed by echocardiography.
Symptomatically, the first patient was suffering mostly from severe dyspnea upon walking 20 m or less due to the pulmonary and cardiac problems mentioned in Table 1. She was also suffering from severe hand and finger scleroderma skin contracture to the point of being unable to hold a cup or feed herself properly holding a spoon or a fork. She was also having joint pain and pain due to Reynaulds phenomenon. Post-therapy, she reported “I got my freedom back”. Her symptoms improved to where she could walk slowly for over 100 m without having to stop even when going upstairs. She became able to fold her fingers more than halfway, hold a spoon to feed herself and hold a cup to drink. Her feeling of independence felt like “her whole life changed to the better”. On the Echocardiogram, her pericardial effusion resolved and her pulmonary pressures normalized.
The second patient had similar dyspnea on mild exertion for less than 20 m before the treatment; she had difficulty with joint movement especially going upstairs and had significant joint pain. Post treatment, she experienced significant relief of her pain and resolution of her pulmonary hypertension by echocardiography. Her motility and ability to exert became “normal” by her account. The relief of her arthritis and Reynaulds phenomenon pain was very significant for her quality of life that she went back to her work as a nurse.
Both patients received a second dose of Allo-MSCs after their symptoms started to progress again but no pulmonary pressure deterioration was noted. The second treatments did not include plasmapheresis or rituximab. Both patients experienced again improvement of their mobility and function (Table 2).
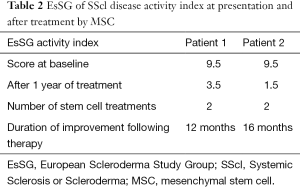
Full table
Discussion
MSCs are believed to act without causing any immune reaction even when given in an allogeneic manner. This idea simulates putting a natural, immune modifying factory that may possibly reset the immune system and reboot it (15,16). Our 2 cases had aggressive, refractory, and debilitating scleroderma that reached a level of irreversible progression. They both benefited from a combination of immunotherapeutic modalities including plasma exchange, rituximab and stem cell therapy. The improvement in their symptoms was especially significant for the resolution of pain, more mobility, less fatigue and dyspnea on mild exertion, and above all more freedom to do what they like.
MSCs were used in both patients when the disease progressed again after the initial protocol. The overall improvement was significant with reduced EsSG activity index as a quantification method. This improvement was also reported subjectively by the patients as improved mobility, function, and quality of life. We believe that the MSCs probably worked through immune modulation to balance the perturbed cytokine and growth factor soup in scleroderma, adjust the immune cellular balance, abate the ongoing fibrosis in scleroderma and therefore changed the course of the disease.
Stem cell therapy is not ready for use on every patient with scleroderma but there are reasons to believe that it is possibly effective where there is indeed no hope to offer. Future large scale studies can bring more scientific evidence to further improve this approach and fulfill the unmet needs to treat advanced scleroderma patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from these two patients for publication of this Case report.
References
- van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737-47. [Crossref] [PubMed]
- Damoiseaux J. Are autoantibodies to RNA-polymerase III to be incorporated in routine diagnostic laboratory algorithms for systemic autoimmune rheumatic diseases? Ann Rheum Dis 2014;73:e29. [Crossref] [PubMed]
- Johnson SR, Goek ON, Singh-Grewal D, et al. Classification criteria in rheumatic diseases: a review of methodologic properties. Arthritis Rheum 2007;57:1119-33. [Crossref] [PubMed]
- Wollheim FA. Classification of systemic sclerosis. Visions and reality. Rheumatology (Oxford) 2005;44:1212-6. [Crossref] [PubMed]
- Jordan S, Distler JH, Maurer B, et al. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis 2015;74:1188-94. [Crossref] [PubMed]
- Singh JA, Solomon DH, Dougados M, et al. Development of classification and response criteria for rheumatic diseases. Arthritis Rheum 2006;55:348-52. [Crossref] [PubMed]
- Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 2013;13:392-402. [Crossref] [PubMed]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007;110:3499-506. [Crossref] [PubMed]
- Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002;30:42-8. [Crossref] [PubMed]
- Klyushnenkova E, Mosca JD, Zernetkina V, et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci 2005;12:47-57. [Crossref] [PubMed]
- Waterman RS, Tomchuck SL, Henkle SL, et al. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One 2010;5:e10088. [Crossref] [PubMed]
- Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 2003;31:890-6. [Crossref] [PubMed]
- Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99:3838-43. [Crossref] [PubMed]
- Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003;57:11-20. [Crossref] [PubMed]
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003;101:3722-9. [Crossref] [PubMed]
- Klyushnenkova E, Mosca JD, McIntosh KR, et al. Human mesenchymal stem cells suppress allogeneic T cell responses in vitro: implications for allogeneic transplantation. Blood 1998;92:2652.
- Yang H. South Korea’s stem cell approval. Nat Biotechnol 2011;29:857. [Crossref]
- Cyranoski D. Canada approves stem cell product. Nat Biotechnol 2011;30:571. [Crossref]
- Ball LM, Bernardo ME, Roelofs H, et al. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol 2013;163:501-9. [Crossref] [PubMed]
- Margossian T, Reppel L, Makdissy N, et al. Mesenchymal stem cells derived from Wharton's jelly: comparative phenotype analysis between tissue and in vitro expansion. Biomed Mater Eng 2012;22:243-54. [PubMed]
Cite this article as: Wehbe T, Abi Saab M, Abi Chahine N, Margossian T. Mesenchymal stem cell therapy for refractory scleroderma: a report of 2 cases. Stem Cell Investig 2016;3:48.


