Maintenance of hematopoietic stem cell dormancy: yet another role for the macrophage
Hematopoietic stem cells (HSC) possess several unique features that ensure a life-long supply of all bone marrow derived blood cell lineages (1). In adulthood, they are mainly localized to the bone marrow and depend on a supportive niche for maintaining their long-term (LT) repopulating ability while simultaneously having a capacity for multi-potent differentiation. The HSC niche has been suggested to be composed of vascular endothelial cells, perivascular mesenchymal stromal cells (2-4), osteoblasts (5) as well as mature hematopoietic cells like megakaryocytes and macrophages (6-9). Furthermore, HSC maintain a low cell cycle activity to prevent exhaustion of their replicative capacity (10). In addition, recent findings suggest that HSC can be subdivided into LT or short-term (ST) HSC of which the latter have a greater propensity for lineage-specific differentiation (11). Mouse LT-HSCs are enriched in a cell population immunophenotypically characterized as Lin− (lineage negative), c-Kit+, Sca1+, CD150+, CD34−, Flk2/Flt3− and CD48− cells. A recent study adds expression of Hoxb5 to these markers for providing additional definition of the LT-HSC population (12).
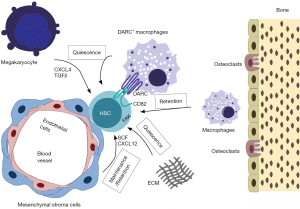
The study by Hur et al. (13) identifies yet another marker for LT-HSC and furthermore ascribes a mechanistic role to this marker. Cd82/Kai1 codes for a membrane receptor (denoted CD82 onwards) belonging to the tetraspanin family of receptors, which interacts with the Darc/Cd234 gene product (denoted Darc onwards) (14). Cd82 is ubiquitously expressed but in the bone marrow, high Cd82 expression is mainly confined to LT-HSC located in endosteal and/or periarteriolar compartments. During hematopoietic differentiation, Cd82/CD82 expression decreases being significantly lower in ST-HSC and multi-potent progenitors. Knockout of Cd82 diminished the population of LT-HSC while simultaneously increasing their proliferation via cell cycle entry. This resulted in a decreased repopulating capacity when competitively transplanted to bone marrow ablated donors, an effect that became increasingly apparent upon performing serial bone marrow transplantations. Surprisingly, this effect was less pronounced when assessing the reconstitution of the myeloid lineage only. Mechanistically, based on in vitro knockdown experiments, CD82 via protein kinase C alpha (PKCa) increases the expression of Tgfb1 and Tgfbr1 that via Smad signaling elevate the expression of cyclin dependent kinase inhibitors (CKI), thus maintaining LT-HSC in a quiescent state.
The cellular source of the CD82 binding partner Darc was next established. Highest bone marrow Darc expression was localized to macrophages that were in direct contact with quiescent LT-HSC. In LT-HSC and Darc+ macrophage co-culture experiments, Darc+ macrophages increased LT-HSC quiescence in a Tgfb1 and Smad3-dependent manner. Additional mechanistic twists delineated in the study were that Darc was required for LT-HSC CD82 expression by prevention of endocytic degradation of CD82 and that Darc-CD82 associations also are operating in maintaining human LT-HSC quiescence.
In summary, a model is proposed suggesting that macrophages via Darc expression increase/retain LT-HSC expression of CD82 and that CD82-signaling via PKCa/Tgfb1/Tgfbr1/Smad3/CKI results in LT-HSC quiescence. Absence of this pathway will eventually deplete the bone marrow of LT-HSC. This pathway may also assist in maintaining physiological hematopoiesis since under physiological steady state conditions, Darc-activated CD82 suppresses LT-HSC proliferation, whereas when the bone marrow is challenged by genotoxic stress, the suppressive effect of CD82 is lost due to the simultaneous disappearance of Darc-expressing macrophages.
The study reveals certain consequences of perturbed bone marrow niche/LT-HSC interactions as a result of CD82 ablation that deserve mentioning. One is that despite increased proliferation, LT-HSC numbers are reduced without a concomitant increase of progenitor populations. Secondly, the loss of lineage repopulation was unequal with a more severe reduction of T and B cell reconstitution than that of myeloid cells. The intricacy in the behavior of LT-HSC in response to aberrant cell cycle regulation can be illustrated by the fact that in two different situations, one due to absence of CD82 (13) and the other due to absence of Shb (15), both revealed reduced numbers of bone marrow LT-HSC and decreased repopulation upon bone marrow reconstitution after transplantation, despite one being due to excessive proliferation and exhaustion of the LT-HSC pool whereas the other was due to increased quiescence caused by cell cycle inhibition. The mechanism behind the reduced proliferation in the absence of Shb is not known but may involve increased expression of the CKI p27Kip1 (Gustafsson and Welsh, unpublished observations), which functionally relates that effect to TGF-beta signaling in LT-HSC. Several recent publications have made a strong case for TGF-beta supporting LT-HSC dormancy and most focus has been on increased expression of the CKIs p21Cip1, p27Kip1 and p57Kip2 via SMAD2/3 activation (16-21). Also of possible relevance is non-canonical signaling inhibiting the PI3-kinase/Akt/FoxO pathway that could exert an effect in this context as well. Taken together, a common denominator in most situations maintaining LT-HSC quiescence could be effects relating to some step in the TGF-beta pathway conferring cell cycle inhibition.
This work also highlights the importance of the bone marrow microenvironment as a protector of LT-HSC integrity. This may, in turn, be one of the main physiological functions of the hematopoietic niche. A number of recent studies suggest that LT-HSC are not the main providers of hematopoiesis under homeostatic conditions in adulthood. Instead it appears as though ST-HSC as well as more differentiated progenitors with self-renewing abilities are the predominant suppliers of new blood cells (11,22), implying that LT-HSC primarily serve as a reservoir in the adult hematopoietic system. In addition, current data suggest that commitment to a specific lineage during the hematopoietic differentiation process, starting with ST-HSC, primarily reflects cell-autonomous features of the relevant cell type (23-26). The primary role of the LT-HSC niche may thus be to enforce quiescence in order to protect the stem cell pool from proliferative exhaustion and accumulation of DNA damage.
Macrophages have already been implicated in LT-HSC regulation through control of mobilization (6,8). We can now add another mechanism, i.e., macrophages and Darc/CD82 signaling, that participates in niche-dependent maintenance of LT-HSC self-renewal. The figure illustrates demonstrated bone marrow niche/LT-HSC interactions that play a role in LT-HSC maintenance (Figure 1).
The role of CD82 in acute myeloid leukemia (AML) has been investigated in a number of studies (29-33). However, none of these relates to high Cd82 expression imposing dormancy on the leukemic cells but rather suggest that CD82 promotes leukemic cell survival and bone marrow homing. This is in contrast to the situation in solid tumors in which CD82 inhibits tumor invasiveness and metastasis, which are effects more in line with the effect on dormancy of LT-HSC (16). The present study stimulates speculation on an additional involvement of CD82 in AML, proposing that leukemic stem cells with high CD82 are more quiescent and thus more resistant to chemotherapy. Future studies will address this important topic.
Acknowledgements
The study was supported by the Swedish cancer foundation, the Family Ernfors foundation, EXODIAB and the Swedish Research Council.
Footnote
Provenance: This is a Guest Commentary commissioned by Editor-in-Chief Zhizhuang Joe Zhao (Pathology Graduate Program, University of Oklahoma Health Sciences Center, Oklahoma City, USA).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014;505:327-34. [Crossref] [PubMed]
- Ding L, Saunders TL, Enikolopov G, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012;481:457-62. [Crossref] [PubMed]
- Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010;466:829-34. [Crossref] [PubMed]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013;495:231-5. [Crossref] [PubMed]
- Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003;425:841-6. [Crossref] [PubMed]
- Chow A, Lucas D, Hidalgo A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 2011;208:261-71. [Crossref] [PubMed]
- Bruns I, Lucas D, Pinho S, et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 2014;20:1315-20. [Crossref] [PubMed]
- Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 2010;116:4815-28. [Crossref] [PubMed]
- Zhao M, Perry JM, Marshall H, et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med 2014;20:1321-6. [Crossref] [PubMed]
- Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet 2008;9:115-28. [Crossref] [PubMed]
- Busch K, Klapproth K, Barile M, et al. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature 2015;518:542-6. [Crossref] [PubMed]
- Chen JY, Miyanishi M, Wang SK, et al. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature 2016;530:223-7. [Crossref] [PubMed]
- Hur J, Choi JI, Lee H, et al. CD82/KAI1 Maintains the Dormancy of Long-Term Hematopoietic Stem Cells through Interaction with DARC-Expressing Macrophages. Cell Stem Cell 2016;18:508-21. [Crossref] [PubMed]
- Bandyopadhyay S, Zhan R, Chaudhuri A, et al. Interaction of KAI1 on tumor cells with DARC on vascular endothelium leads to metastasis suppression. Nat Med 2006;12:933-8. [Crossref] [PubMed]
- Gustafsson K, Heffner G, Wenzel PL, et al. The Src homology 2 protein Shb promotes cell cycle progression in murine hematopoietic stem cells by regulation of focal adhesion kinase activity. Exp Cell Res 2013;319:1852-64. [Crossref] [PubMed]
- Liu WM, Zhang XA. KAI1/CD82, a tumor metastasis suppressor. Cancer Lett 2006;240:183-94. [Crossref] [PubMed]
- Yamazaki S, Ema H, Karlsson G, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 2011;147:1146-58. [Crossref] [PubMed]
- Matsumoto A, Takeishi S, Kanie T, et al. p57 is required for quiescence and maintenance of adult hematopoietic stem cells. Cell Stem Cell 2011;9:262-71. [Crossref] [PubMed]
- Zou P, Yoshihara H, Hosokawa K, et al. p57(Kip2) and p27(Kip1) cooperate to maintain hematopoietic stem cell quiescence through interactions with Hsc70. Cell Stem Cell 2011;9:247-61. [Crossref] [PubMed]
- Blank U, Karlsson S. TGF-β signaling in the control of hematopoietic stem cells. Blood 2015;125:3542-50. [Crossref] [PubMed]
- Quéré R, Saint-Paul L, Carmignac V, et al. Tif1γ regulates the TGF-β1 receptor and promotes physiological aging of hematopoietic stem cells. Proc Natl Acad Sci U S A 2014;111:10592-7. [Crossref] [PubMed]
- Sun J, Ramos A, Chapman B, et al. Clonal dynamics of native haematopoiesis. Nature 2014;514:322-7. [Crossref] [PubMed]
- Paul F, Arkin Y, Giladi A, et al. Transcriptional Heterogeneity and Lineage Commitment in Myeloid Progenitors. Cell 2015;163:1663-77. [Crossref] [PubMed]
- Perié L, Duffy KR, Kok L, et al. The Branching Point in Erythro-Myeloid Differentiation. Cell 2015;163:1655-62. [Crossref] [PubMed]
- Sanjuan-Pla A, Macaulay IC, Jensen CT, et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature 2013;502:232-6. [Crossref] [PubMed]
- Yamamoto R, Morita Y, Ooehara J, et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 2013;154:1112-26. [Crossref] [PubMed]
- Lu J, Sun Y, Nombela-Arrieta C, et al. Fak depletion in both hematopoietic and nonhematopoietic niche cells leads to hematopoietic stem cell expansion. Exp Hematol 2012;40:307-17.e3. [Crossref] [PubMed]
- Gustafsson K, Jamalpour M, Trinh C, et al. The Src homology-2 protein Shb modulates focal adhesion kinase signaling in a BCR-ABL myeloproliferative disorder causing accelerated progression of disease. J Hematol Oncol 2014;7:45. [Crossref] [PubMed]
- Burchert A, Notter M, Dietrich Menssen H, et al. CD82 (KAI1), a member of the tetraspan family, is expressed on early haemopoietic progenitor cells and up-regulated in distinct human leukaemias. Br J Haematol 1999;107:494-504. [Crossref] [PubMed]
- Marjon KD, Termini CM, Karlen KL, et al. Tetraspanin CD82 regulates bone marrow homing of acute myeloid leukemia by modulating the molecular organization of N-cadherin. Oncogene 2016;35:4132-40. [Crossref] [PubMed]
- Nishioka C, Ikezoe T, Furihata M, et al. CD34+/CD38− acute myelogenous leukemia cells aberrantly express CD82 which regulates adhesion and survival of leukemia stem cells. Int J Cancer 2013;132:2006-19. [Crossref] [PubMed]
- Nishioka C, Ikezoe T, Takeuchi A, et al. The novel function of CD82 and its impact on BCL2L12 via AKT/STAT5 signal pathway in acute myelogenous leukemia cells. Leukemia 2015;29:2296-306. [Crossref] [PubMed]
- Nishioka C, Ikezoe T, Yang J, et al. CD82 regulates STAT5/IL-10 and supports survival of acute myelogenous leukemia cells. Int J Cancer 2014;134:55-64. [Crossref] [PubMed]
Cite this article as: Gustafsson K, Welsh M. Maintenance of hematopoietic stem cell dormancy: yet another role for the macrophage. Stem Cell Investig 2016;3:46.


