From stem cells to comparative corticogenesis: a bridge too far?
Introduction
Despite a wealth of recent influential studies addressing the specific features of the human gene regulatory network of corticogenesis (1-7), the question of what makes us human is still unresolved. The human cerebral cortex has achieved a unique degree of complexity considered to underlie the emergence of unrivaled cognitive abilities compared to other species including non-human primates (8,9). One level of explanation is that the human brain contains a larger number of neurons (10), particularly in supragranular layers where they form a dense core of cortico-cortical connections (11). Quantitative studies of the number of neurons in brains of different weights (12) indicate species-specific scaling rules. For a given brain weight, compared to rodents, primate cortices accommodate more neurons suggesting qualitative differences in corticogenesis between the two clades. Major differences in cortical development between rodents and primates are observed in the organization and cell lineages of the germinal zones with the large expansion of the subventricular zone (SVZ) that is divided into an inner and most significantly outer SVZ in primates [OSVZ, (13) in macaque; (14,15) in human]. Significant primate-rodent differences have also been observed in the cell identity of cortical progenitor types; primate corticogenesis is characterized by a more diverse repertoire of cortical progenitors with uniquely high proliferative potential and non-hierarchical lineage relationships (14-18).
Numerous primate specificities in addition to the size of the OSVZ include a high percentage of basal radial glial cells (bRGs) (14,15,18). The human cortex contains twice and ten as many neurons as in chimpanzee and macaque monkey respectively (19). Nevertheless macaque and human follow a common scaling law which is distinct for that observed in rodents (20). This strengthens the hypothesis that the differences in neuron production between human and other non-human primates could be merely quantitative, due to differences in the timing of the neurogenic period or in its duration for instance (21,22). Investigating the specificities of human corticogenesis compared to other primate species is technically challenging. In the recent study by Otani et al., Cell Stem Cell 2016 the authors take advantage of in vitro corticogenesis culture systems to directly investigate possible differences between human, chimpanzee and macaque monkey early cortical development (23).
Early attempts in vitro corticogenesis led to high hopes to model normal development and diseases (24-28), especially in humans where access to fresh and viable tissue is notoriously difficult (6,15). Recently, significant advances have been achieved due to the development of protocols allowing the generation of human neural rosettes (27), and human cortical organoids (25,26). These models corresponding to 2 and 3D culture systems take advantage of the remarkable self-constructing properties of biological tissues. When grown in appropriate conditions, in a 3D environment or in the presence of the relevant factors, pluripotent stem cells capture certain features of corticogenesis, providing potentially useful models of in vitro human cortical development. The protocol established by Shi et al. on human pluripotent stem cells, allows the sequentially ordered generation of diverse cortical neuron types in cortical rosettes (27). Otani and collaborators have exploited this protocol to produce cortical rosettes from macaque and chimpanzee stem cells, thereby allowing comparison of corticogenesis in vitro in these three primate species (23).
In vitro differences between human, chimpanzee and macaque cortical development
Building on the protocol developed in Shi et al. 2012 to produce cortical rosettes from human pluripotent stem cells, Otani et al. succeeded in generating cortical rosettes from chimpanzee and macaque pluripotent stem cells. Sixty days in culture allowed a recapitulation of the in vivo temporal sequence: an “infragranular” (IG) layer component expressing Tbr1 (layers VI and V), followed by a “supragranular” (SG) layer component expressing Satb2 (layers IV, III and II). The cortical rosettes neurons so produced migrate in an inside-out in vivo like fashion forming a ‘cortical plate’ that displays some laminated organization, reminiscent of corticogenesis in vivo [Figure 2C in (23)].
The culture system recapitulates to a certain extent the composition of the germinal zones of the different species with densely packed radial glial cells (RGC) expressing the transcription factor Pax6 (14,15,18). The RGC display apical junctions at the apical side lining a lumen, reminiscent of and referred to as the in vitro ventricular zone (VZ). The authors report some Tbr2 expressing basal progenitors located on top of the in vitro VZ. They also describe a few abventricular Pax6 expressing cells some of which display the characteristic of basal radial glia with a basal process (bRG-basal-P, also referred to as bRG or oRG) (14,15,18).
Using this in vitro corticogenesis system, Otani and colleagues report differences in cortical development between the three primate species. These differences appear to be related to changes in the timing of developmental events, a process referred to as heterochrony (29,30). Compared to human and chimpanzee, macaque cortical precursors are reported to terminate their expansion phase earlier and switch precociously from IG to SG neuron production (Figure 1). Interestingly, the maturation period of macaque neurons appears shorter than that observed in human and chimpanzee.
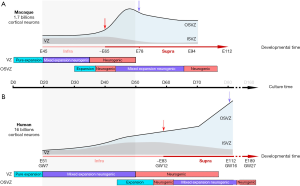
Species-specific differences in the timing of precursor pool dynamics in vitro?
A central aspect of the Otani and colleagues’ study is a clonal analysis of cortical precursors labeled by lentiviral infection at different culture time points. This permits monitoring the dynamics of the precursor population, making it possible to analyze key parameters controlling neuron production rates (21) (cell cycle length, incidence of cell death, frequency of cell cycle exit, division pattern) over a 20-day period, from the 20th day of neural differentiation (D20) to D40.
Importantly the in vitro precursor dynamics of the three primate species differ markedly from mouse corticogenesis (33). By contrast to the mouse corticogenesis, the in vitro cultures in all three primates showed expansion of the precursor pool after the onset of neurogenesis as observed in vivo (18,34) (Figure 1). This mixed proliferative/neurogenic phase is more prolonged in human compared to macaque cultures. The macaque precursor dynamics were found to differ from the human precursors’ from D30 onwards. Macaque clones were significantly smaller during the peak of IG neuron generation. The lineage trees in human and macaque rosettes at this crucial time point showed macaque precursors undergoing less frequent symmetric proliferative divisions and more frequent terminal neurogenic divisions leading to an exhaustion of the macaque precursor pool. In human cortical rosettes, the precursor pool expands for a longer period than in the macaque cultures, despite the peak of IG neuron production. These results point to heterochrony in in vitro corticogenesis between the three different primate species.
The in vitro culture accurately models the early stages of corticogenesis
An extensive and reliable database of the macaque cortical precursor proliferation dynamics has been provided by a series of in vivo and ex vivo studies (18,31,34-38). Establishing the correspondence between the in vitro and the in vivo tempo of corticogenesis is a prerequisite to fully appraise the relevance of the in vitro findings. The above described results of the precursor pool dynamics from Otani et al. have been obtained from observations over a 20-day period, from D20 to D40 in macaque and human cultures. D40 in macaque cultures corresponds to the end of layer V, beginning of layer IV production [circa E65 in the macaque visual cortex (31,32,36)] and to the peak of layer VI production in human [circa E65 in the human visual cortex (32)] (Figure 1). The 20-day culture period corresponds therefore to the end IG layer generation in macaque and the peak of IG neuron production in human.
The macaque in vitro culture system recapitulates accurately the behavior of native macaque cortical precursors at the early developmental stages, as evidenced by the changes reported in macaque precursor behavior (D35)—corresponding to circa E65 in the in vivo macaque (end of layer V production). At E65 ex vivo live-imaging of cortical precursors showed that there is a majority of neurogenic and only few symmetric proliferative divisions (30%) both in the VZ and the OSVZ (18), a behavior also found in macaque cortical rosettes.
Limitations of the model command caution in interpretation - the need to consider interspecies heterochrony
The clonal analysis performed between D20 and D50 (D40+10 days survival) revealed differences in precursor pool dynamics between macaque and human, showing a switch from a mixed expansion/neurogenic to a mainly neurogenic/exhaustion phase in the macaque, whereas human precursors remain in the mixed expansion/neurogenic phase (23). These findings suggest that the in vitro system faithfully captures in vivo heterochrony: the earlier peak of IG neuron production in the macaque. As expected for cortical precursors in expansion vs. neurogenic phases (18), Otani et al. found that the proliferative abilities of macaque and human precursors at D30 are different. However, this does not necessarily reflect “interspecies difference in clonal expansion” as stated page 472 (23). Testing such interspecies difference in clonal expansion would require analysis clones at equivalent developmental stages; the macaque in vitro D40 equivalent stage in human would be circa D60 (Figure 1)—a stage which has not yet been explored by Otani et al.
Do in vitro systems reproduce the complex dynamics of primate OSVZ precursors?
Cortical organotypic slice culture and live-imaging has provided important insights into the primate-specific features of the precursor pool dynamics (18). This work revealed that the IG precursor pool was characterized by a longer cell-cycle duration and a high frequency of neurogenic divisions, both of which result in a limited expansion (39). By contrast, the later SG OSVZ precursor pool showed shorter cell-cycle duration as well as extensive proliferative abilities which, combined with less frequent neurogenic divisions results in a rapid expansion of the OSVZ (18) (Figure 1).
The ex vivo results from Betizeau et al. are in agreement with in vivo observations. The early and exhaustive in vivo birth-dating studies of macaque corticogenesis by the group of Pasko Rakic have shown that the tempo of cell-cycle regulation in the primate largely departs from that of the mouse (34), which has been confirmed by recent work in the ferret (40). After a period of expansion characterized by a short cell cycle length (Tc) of VZ precursors [~E40 macaque (18,34)], cortical precursors shift towards a mainly neurogenic period at the time of IG neuron generation characterized by a longer Tc both in the VZ and the OSVZ (~E60). The upsurge of proliferation observed at the beginning of SG neuron production in the macaque monkey (~E80) is correlated with a shorter Tc of VZ and OSVZ precursors (18,34) (Figure 1). A similar shortening of Tc during the SG neuron production has also been observed in the ferret (40).
Is this upsurge of proliferation during the generation of SG neurons reproduced in cultures? Given that the timing of developmental events is delayed in human corticogenesis, such a change in precursor behavior is predicted to occur at GW12 in vivo (Figure 1) (32), corresponding to around D60 in vitro, a stage that has not been explored by Otani et al. Further work is therefore required to explore the dynamics of cortical precursors during the generation of SG neurons in vitro.
3D does not suffice to recreate the OSVZ niche
The OSVZ has been shown to be a key player in cortical complexification and in the radial expansion of the primate cerebral cortex (14,15,17,18,37). The OSVZ is a niche fostering stemness, thereby allowing expansion of the precursor pool (6,41). Despite evidence of delamination in a fraction of cortical rosette precursors, there is a failure to form an OSVZ proper with its dense architecture, increased thickness and expanded basal progenitor pool. Moreover, there is no mention of the morphological diversity of basal progenitors that is characteristic of the primate OSVZ. In theory, for this in vitro culture model to constitute an appropriate tool to explore primate corticogenesis, it would have to recapitulate the full repertoire of cortical progenitors observed in macaque monkey and human organotypic slice cultures (16,18,42,43). An in vitro model which faithfully recapitulates the later stages of corticogenesis with a prominent OSVZ (Figure 1) would provide the unique opportunity to probe emergence of precursor diversity and its variation in the primate lineage. It would also allow an in-depth analysis of the intriguing non-hierarchical lineages, which include bi-directional transitions—that characterize OSVZ precursors (18,43) and which are not observed in non-primates (44-46). The recent culture system described by Qian and colleagues (47), where an OSVZ like structure is seen to emerge and precursors express bRG specific markers (6), might offer this exciting possibility.
Towards better in vitro modeling of primate late corticogenesis
The timing correspondence between in vivo and in vitro development established above showed that the time points examined in the in vitro model only span through the early stages of corticogenesis (Figure 1). Together with the in vivo heterochrony between human and macaque corticogenesis, this limitation underlies the need to probe a wider range of in vitro time points, in order to address physiologically relevant issues.
Primate brains are characterized by an expansion of the cortex but this expansion did not occur isotropically. The SG layer compartment has been specifically enlarged containing 66% and 60% of the cortical neurons in human and macaque respectively compared to 49% in the mouse (48). This primate specific feature has to be reproduced in culture systems in order to model potential defects relative to SG neurons (49). In the study by Otani et al, at the latest culture stage (D70) macaque rosettes are composed of maximum 50% of SGN, and human rosettes of only around 25%. This points to the current limitations of the culture system especially in the human case. Technical issues prevent cultures to achieve the late developmental stages. This could be related to perfusion problems of the structure which becomes too large and compromise survival and normal development. The explanation could be linked to the very limited basal progenitor pool and the quasi absence of OSVZ, responsible for the generation of the majority of SG neurons (37).
Further developing the cultures would require either improving the survival of larger structures by a better vascularization or finding a way of speeding up corticogenesis (28). The new culture system developed by Qian and colleagues goes further in this direction and seem to overcome these hurdles allowing the development of a more significant OSVZ and the generation of later-born neurons (47). Future in vitro studies in this direction will undoubtedly give us more insights into these primate specific features of corticogenesis.
Heterochrony is cell autonomous
Lastly, the authors performed elegant experiments to determine whether the observed differences between human and macaque precursors are cell autonomous or dictated by their environment (23). Would macaque or human precursors behave differently placed in a different environment? To address this issue, macaque precursors of D35 were “grafted” into human rosettes of the same culture time and vice versa. Surprisingly, macaque and human precursors continue behaving as in their native environment. Human precursors continued to expand, producing mainly IG layer neurons in an environment where macaque precursors already switched to more neurogenic divisions and to the production of SG layers. Moreover, macaque precursors did not switch back to an expansion phase but instead continued producing SG layer neurons when co-cultured in human cortical rosette. The dynamics of cortical precursors in human and macaque thus seems controlled cell-autonomously, suggesting that the heterochrony of developmental event is controlled at the genetic level. This human specific control could be due to genetic mutation in the human lineage (7) as illustrated by the gene Arhgap11b arisen from a duplication specific to the human lineage and involved in cortical expansion (3). This cell-autonomous control of precursor dynamics could also be due to specific regulation of gene expression [lnRNA (50,51); miRNA (52); different timings of gene expression (1,6,53)].
Conclusions
It is commonly stated that all models are wrong but some are useful (54). The model proposed by Otani et al. shows just how profitable is the in-depth comparative analysis of primate precursor pool dynamics in cortical organoids. Differences mainly point to the need to better understand heterochrony of developmental events, especially the prolonged mixed phase of expansion and IG generation in human, which could eventually contribute to the larger number of neurons produced in humans. Extending the in vitro models to late corticogenesis is expected to bring additional insight into what makes us human.
Acknowledgements
We thank Henry Kennedy and Delphine Delaunay for fruitful discussions.
Funding: This work was supported by the LabEx CORTEX (ANR-11-LABX-0042) and LabEx DEVweCAN (ANR-10-LABX-0061) of Université de Lyon (ANR-11-IDEX-0007) operated by the French National Research Agency, ANR-10-IBHU-0003 (IHU CESAME), ANR-14-CE13-0036 (Primacor) and the Fondation pour la Recherche Médicale (ING20130526653). M Betizeau is funded by SystemsX.ch (Transition Postdoc fellowship).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Camp JG, Badsha F, Florio M, et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U S A 2015;112:15672-7. [PubMed]
- Charrier C, Joshi K, Coutinho-Budd J, et al. Inhibition of SRGAP2 function by its human-specific paralogs induces neoteny during spine maturation. Cell 2012;149:923-35. [Crossref] [PubMed]
- Florio M, Albert M, Taverna E, et al. Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. Science 2015;347:1465-70. [Crossref] [PubMed]
- Johnson MB, Kawasawa YI, Mason CE, et al. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron 2009;62:494-509. [Crossref] [PubMed]
- Parikshak NN, Gandal MJ, Geschwind DH. Systems biology and gene networks in neurodevelopmental and neurodegenerative disorders. Nat Rev Genet 2015;16:441-58. [Crossref] [PubMed]
- Pollen AA, Nowakowski TJ, Chen J, et al. Molecular identity of human outer radial glia during cortical development. Cell 2015;163:55-67. [Crossref] [PubMed]
- Sudmant PH, Kitzman JO, Antonacci F, et al. Diversity of human copy number variation and multicopy genes. Science 2010;330:641-6. [Crossref] [PubMed]
- Deaner RO, Isler K, Burkart J, et al. Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain Behav Evol 2007;70:115-24. [Crossref] [PubMed]
- Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron 2013;80:633-47. [Crossref] [PubMed]
- Roth G, Dicke U. Evolution of the brain and intelligence. Trends Cogn Sci 2005;9:250-7. [Crossref] [PubMed]
- Markov NT, Ercsey-Ravasz M, Van Essen DC, et al. Cortical high-density counterstream architectures. Science 2013;342:1238406. [Crossref] [PubMed]
- Herculano-Houzel S. Not all brains are made the same: new views on brain scaling in evolution. Brain Behav Evol 2011;78:22-36. [Crossref] [PubMed]
- Smart IH, Dehay C, Giroud P, et al. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex 2002;12:37-53. [Crossref] [PubMed]
- Fietz SA, Kelava I, Vogt J, et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci 2010;13:690-9. [Crossref] [PubMed]
- Hansen DV, Lui JH, Parker PR, et al. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 2010;464:554-561. [Crossref] [PubMed]
- Florio M, Huttner WB. Neural progenitors, neurogenesis and the evolution of the neocortex. Development 2014;141:2182-94. [Crossref] [PubMed]
- Dehay C, Kennedy H, Kosik KS. The outer subventricular zone and primate-specific cortical complexification. Neuron 2015;85:683-94. [Crossref] [PubMed]
- Betizeau M, Cortay V, Patti D, et al. Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron 2013;80:442-57. [Crossref] [PubMed]
- Herculano-Houzel S, Collins CE, Wong P, et al. Cellular scaling rules for primate brains. Proc Natl Acad Sci U S A 2007;104:3562-7. [Crossref] [PubMed]
- Azevedo FA, Carvalho LR, Grinberg LT, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 2009;513:532-41. [Crossref] [PubMed]
- Cahalane DJ, Charvet CJ, Finlay BL. Modeling local and cross-species neuron number variations in the cerebral cortex as arising from a common mechanism. Proc Natl Acad Sci U S A 2014;111:17642-7. [Crossref] [PubMed]
- Lewitus E, Kelava I, Kalinka AT, et al. An adaptive threshold in mammalian neocortical evolution. PLoS Biol 2014;12:e1002000. [Crossref] [PubMed]
- Otani T, Marchetto MC, Gage FH, et al. 2D and 3D stem cell models of primate cortical development identify species-specific differences in progenitor behavior contributing to brain size. Cell Stem Cell 2016;18:467-80. [Crossref] [PubMed]
- Eiraku M, Watanabe K, Matsuo-Takasaki M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 2008;3:519-32. [Crossref] [PubMed]
- Kadoshima T, Sakaguchi H, Nakano T, et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A 2013;110:20284-9. [Crossref] [PubMed]
- Lancaster MA, Renner M, Martin CA, et al. Cerebral organoids model human brain development and microcephaly. Nature 2013;501:373-9. [Crossref] [PubMed]
- Shi Y, Kirwan P, Smith J, et al. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci 2012;15:477-86, S1.
- Kelava I, Lancaster MA. Stem cell models of human brain development. Cell Stem Cell 2016;18:736-48. [Crossref] [PubMed]
- Gould SJ. Ontogeny and phylogeny. Cambridge: Harvard University Press; 1977.
- Kennedy H, Dehay C. Cortical specification of mice and men. Cereb Cortex 1993;3:171-86. [Crossref] [PubMed]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science 1974;183:425-7. [Crossref] [PubMed]
- Workman AD, Charvet CJ, Clancy B, et al. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci 2013;33:7368-83. [Crossref] [PubMed]
- Gao P, Postiglione MP, Krieger TG, et al. Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell 2014;159:775-88. [Crossref] [PubMed]
- Kornack DR, Rakic P. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proc Natl Acad Sci U S A 1998;95:1242-6. [Crossref] [PubMed]
- Cunningham CL, Martínez-Cerdeño V, Noctor SC. Diversity of neural precursor cell types in the prenatal macaque cerebral cortex exists largely within the astroglial cell lineage. PLoS One 2013;8:e63848. [Crossref] [PubMed]
- Dehay C, Giroud P, Berland M, et al. Modulation of the cell cycle contributes to the parcellation of the primate visual cortex. Nature 1993;366:464-6. [Crossref] [PubMed]
- Lukaszewicz A, Savatier P, Cortay V, et al. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron 2005;47:353-64. [Crossref] [PubMed]
- Zilles K, Werners R, Büsching U, et al. Ontogenesis of the laminar structure in areas 17 and 18 of the human visual cortex. A quantitative study. Anat Embryol (Berl) 1986;174:339-53. [Crossref] [PubMed]
- Pilaz LJ, Patti D, Marcy G, et al. Forced G1-phase reduction alters mode of division, neuron number, and laminar phenotype in the cerebral cortex. Proc Natl Acad Sci U S A 2009;106:21924-9. [Crossref] [PubMed]
- Reillo I, Borrell V. Germinal zones in the developing cerebral cortex of ferret: ontogeny, cell cycle kinetics, and diversity of progenitors. Cereb Cortex 2012;22:2039-54. [Crossref] [PubMed]
- Fietz SA, Lachmann R, Brandl H, et al. Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix in progenitor self-renewal. Proc Natl Acad Sci U S A 2012;109:11836-41. [Crossref] [PubMed]
- LaMonica BE, Lui JH, Hansen DV, et al. Mitotic spindle orientation predicts outer radial glial cell generation in human neocortex. Nat Commun 2013;4:1665. [Crossref] [PubMed]
- Pfeiffer M, Betizeau M, Waltispurger J, et al. Unsupervised lineage-based characterization of primate precursors reveals high proliferative and morphological diversity in the OSVZ. J Comp Neurol 2016;524:535-63. [Crossref] [PubMed]
- Noctor SC, Martínez-Cerdeño V, Ivic L, et al. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 2004;7:136-44. [Crossref] [PubMed]
- Pilz GA, Shitamukai A, Reillo I, et al. Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type. Nat Commun 2013;4:2125. [Crossref] [PubMed]
- Tyler WA, Haydar TF. Multiplex genetic fate mapping reveals a novel route of neocortical neurogenesis, which is altered in the Ts65Dn mouse model of Down syndrome. J Neurosci 2013;33:5106-19. [Crossref] [PubMed]
- Qian X, Nguyen HN, Song MM, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 2016;165:1238-54. [Crossref] [PubMed]
- Hutsler JJ, Lee DG, Porter KK. Comparative analysis of cortical layering and supragranular layer enlargement in rodent carnivore and primate species. Brain Res 2005;1052:71-81. [Crossref] [PubMed]
- Yu TW, Mochida GH, Tischfield DJ, et al. Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat Genet 2010;42:1015-20. [Crossref] [PubMed]
- Liu SJ, Nowakowski TJ, Pollen AA, et al. Single-cell analysis of long non-coding RNAs in the developing human neocortex. Genome Biol 2016;17:67. [Crossref] [PubMed]
- Rani N, Nowakowski TJ, Zhou H, et al. A primate lncRNA mediates notch signaling during neuronal development by sequestering miRNA. Neuron 2016;90:1174-88. [Crossref] [PubMed]
- Arcila ML, Betizeau M, Cambronne XA, et al. Novel primate miRNAs coevolved with ancient target genes in germinal zone-specific expression patterns. Neuron 2014;81:1255-62. [Crossref] [PubMed]
- Okamoto M, Miyata T, Konno D, et al. Cell-cycle-independent transitions in temporal identity of mammalian neural progenitor cells. Nat Commun 2016;7:11349. [Crossref] [PubMed]
- Box GE, Draper NR. Empirical model-building and response surfaces. New York: Wiley, 1987.
Cite this article as: Betizeau M, Dehay C. From stem cells to comparative corticogenesis: a bridge too far? Stem Cell Investig 2016;3:39.


